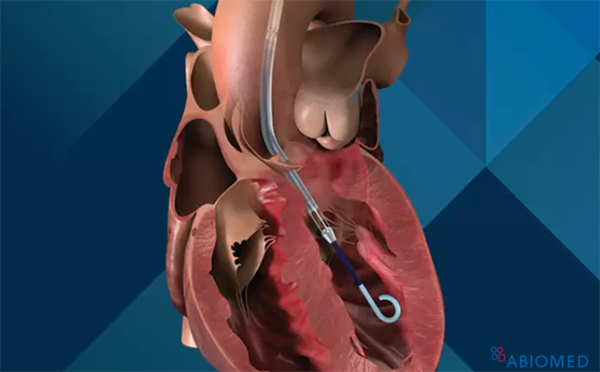
Ventricular assist devices (VADs) are a group of implantable devices that assist and support a failing left or right ventricle.
They come in various forms, including but not limited to surgically implanted devices such as the CentriMag® VAD by Abbot Labs, the intra-aortic balloon pump (IABPs) made by multiple manufacturers, as well as Impella® heart pumps developed by Abiomed Inc.
Surgically placed IABPs were developed in the 1960s and 1970s to treat cardiogenic shock. Technology has improved, and since the 1980s, IABPs have been placed percutaneously for a wider breadth of applications, including high-risk percutaneous coronary intervention (PCI) and post-acute myocardial infarction (AMI). Recently, however, more data has been published questioning the clinical utility of IABP in patients with AMI-related cardiogenic shock (AMI-CS). Momentum to mitigate the mortality of this sickly patient population has led to the development of more forms of mechanical circulatory support. In this article we will be discussing in depth the Impella® device, which was originally approved in 2008 by the Federal Drug Administration.1
What Is an Impella?
The Impella® catheter system can be placed either percutaneously or surgically in the axillary or femoral arterial systems. It is placed across the aortic valve and uses an impeller to draw blood from the LV and pump it into the aorta. There are a few versions of the device, and depending on which one is chosen, they can provide 2-6 L/min of cardiac output. With this support, the left ventricle end-diastolic volume (EDV) is reduced, improving the intrinsic contractility of the myocardium and reducing overall myocardial oxygen demand.2 Furthermore, the large volume of blood delivered to the aorta improves mean arterial pressure and cardiac output, which in turn improves systemic and coronary perfusion.2 Not only does the Impella® benefit the left side of the heart, but also it results in a decreased pulmonary capillary wedge pressure and decrease in right ventricular afterload.2
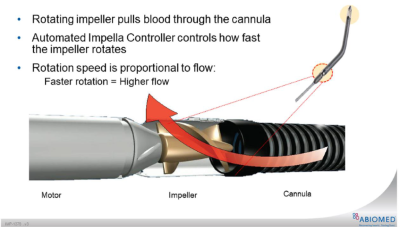
Fig. 1. Illustration of forward flow generated by rotational speed of the impeller, pulling the blood from the LV into the ascending aorto (image from Abiomed)
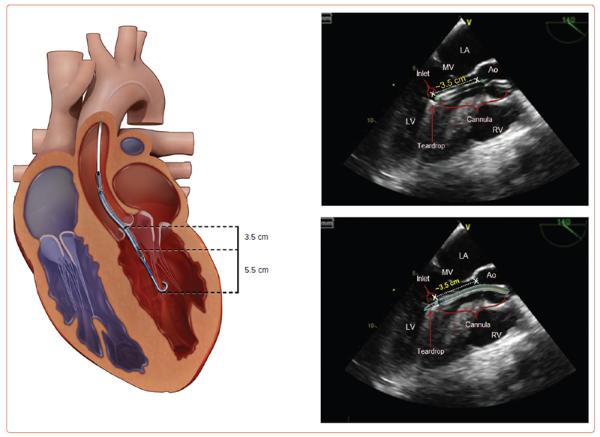
Fig 2. Correct positiong of the Impella® CP, Impella® 2.5, and Impella® 5.0 across the aortic valve and into the left ventricle. The radiopaque marker should be positioned across the aortic valve annulus, allowing an approximate distance of 3.5 cm from the aortic valve annulus to mid-inlet for the Impella® devices (left). The device extends a further 5.5 cm from mid-inlet to the tip of the pigtail catheter. On transthoracic echo (TTE), 2 echogenic double lines of the cannula indicate either end of the Impella® inlet. Reverberation artifacts on TTE posterior to the cannula also may assist in identification of the inlet. Correct placement of the Impella® 5.5 catheter is 5.5 cm from the aortic valve annulus to mid-inlet of the inflow cage. This is deeper due to the lack of pigtail on the Impella® 5.5 catheter. Ao = aorta; LV = left ventricle; MV = mitral valve; RV = right ventricle.
Image credit: Rami Zein, Chirdeep Patel, Adrian Mercado-Alamo, Theodore Schreiber, Amir Kaki, for Interventional Cardiology 2022;17:e05.
Indications
When assessing a patient in cardiogenic shock, it is worth assessing the degree and planned trajectory of their shock state. Early multidisciplinary discussions with specialists in advanced heart failure, interventional cardiology, cardiothoracic surgery, and others will help guide appropriate decision-making for an unwaveringly difficult patient population to treat. General indications for implementation of mechanical circulatory support (MCS) are low or dropping cardiac index below 2.2L/min/m^2, systolic pressures below 90 mmHg, PCWP over 20 mmHg, and evidence of end organ perfusion dysfunction.3 Within this mental framework, patients can be assessed on the Interagency Registry of Mechanically Assisted Circulatory Support (INTERMACS) Patient Profile scale. The INTERMACS scale ranges from 1 to 7, with 1 being the most critically ill patients who are crashing on maximal inotropic support and requiring mechanical circulatory support, and 7 being NYHA Class III patients who are comfortable but limited to mild activity and exertion. In the critically ill cardiac patients, planning for possible MCS intervention typically does and should enter the conversation when patients start bridging from INTERMACS 3 to 2; demonstrating worsening cardiac function despite chemical inotropic support.4
- Acute Myocardial Infarction - Cardiogenic Shock (AMI-CS)
- For many years cardiologists and cardiac surgeons alike were using the IABP as their preferred left ventricular assist device in patients with cardiogenic shock. That is until the IABP-SHOCK II trial showed no mortality benefit in AMI-CS patients.5 It is proposed that the superior hemodynamic benefits of the Impella® over the IABP should lead to improved patient outcomes and a decrease in overall mortality in this patient population. The USpella registry also showed that patients suffering from AMICS had considerably lower mortality when Impella® was placed prior to PCI.6 The DanGer Shock trial published in 2024 evaluated the use of the impella microaxial flow pump vs. standard of care in infarct-related cardiogenic shock. Investigators found the routine use of Impella® in this scenario significantly reduced the risk of death from any cause at 180 days.7
- High-Risk Percutaneous Intervention (high-risk PCI)
- Patients with multivessel or left main coronary artery disease are often referred for surgical evaluation for revascularization via coronary artery bypass grafting. Unfortunately, many of these patients have a complex medical history with multiple medical comorbidities, making them poor surgical candidates. This leads to consideration for a high-risk PCI rather than standard surgical intervention. The concern exists that the transient ischemia induced by stent or balloon inflation can lead to prompt hemodynamic collapse. Multiple studies have supported the positive hemodynamic benefit of implementing early Impella® placement in these patients6 and, when compared to implementation of IABP, Impella® has led to fewer adverse events with a trend toward improved clinical outcomes.8
- Persistent Malignant Arrhythmias
- Anywhere from 50-80% of patients with VT secondary to structural heart disease referred to electrophysiology for ablation have unstable VT, which can cause considerable hemodynamic compromise or collapse when performing the ablation.9 The PERMIT-I trial showed that those patients who had an Impella® placed prior to VT ablation had more favorable hemodynamics than the cohort who received pharmacologic intervention alone.10
Contraindications
- Left Ventricular Thrombus
- Thrombus in the left ventricle poses a number of risks, chiefly the possibility of the thrombus clogging the impeller itself or the clot being broken up by the pump and showering emboli throughout the systemic circulation.
- Severe Aortic Regurgitation (AR)
- Impella® has the potential to worsen AR, as it can increase the regurgitant volume across the valve. However, some case studies have shown Impella® to be a viable bridge to definitive surgical therapy in patients with severe AR and MR.11 For this reason, careful consideration is required, and the decision should be made with a multidisciplinary approach.
- Severe Aortic Stenosis (AS)
- Severe aortic stenosis with a valve area <0.6 cm2 has long been considered a contraindication for Impella® However, some newer studies have shown that Impella® placement is both feasible and safe in patients undergoing high-risk PCI and transcatheter aortic valve replacement (TAVR), which may open the door for further evaluation of its utility in this patient population.12 Careful consideration is required, and the decision should be made with a multidisciplinary approach.
- Severe Peripheral Vascular Disease
- Severe peripheral vascular disease may preclude placement of the catheter at the femoral access site due to vascular calcification or stenosis; however, this can usually be overcome by taking the axillary approach for catheter insertion. Careful consideration is required, and the decision should be made with a multidisciplinary approach.
- Aortic dissection
- Mechanical aortic valve
- Significant right heart failure
- Left ventricular rupture
- Cardiac tamponade
Overview of Impella Devices
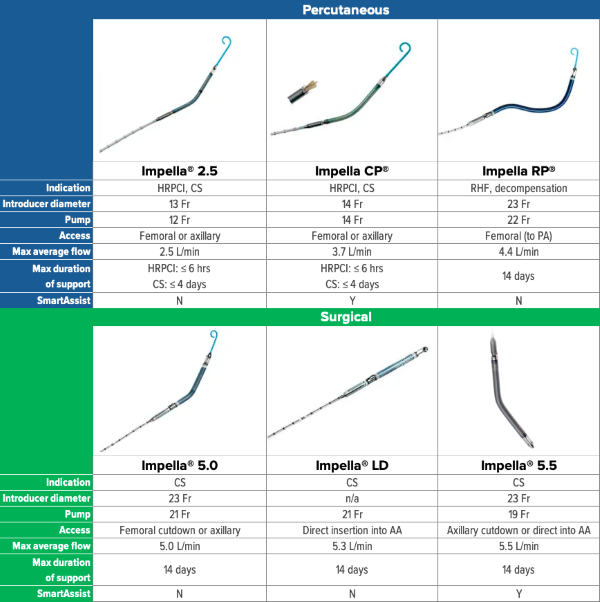
Fig. 3. Overview
Fig. 4. Impella® controller (image courtesy of Abiomed)
Mechanism/ Pathophysiology
The Impella® device is placed percutaneously or via surgical cutdown and is a continuous flow pump that transverses the aortic valve. The “drainage” occurs from the left ventricle and is pumped into the aorta within the pump chamber. Inherently, this makes the pump preload dependent and afterload sensitive. For this reason, maximum pump function and flow are achieved during systole when the gradient between the LV and aorta is at its lowest.
The pump has various levels of flow achieved based on the settings specified by the operator. P1 through P9, with P1 providing minimal support and P9 providing maximal support. The forward flow capabilities vary across the different models (see Fig. 3). Afterload effect becomes apparent when patients are on high-dose vasopressors and the Impella® simultaneously. This will affect the function of the Impella® and may require increasing support levels to overcome the resistance of the systemic circulation. In theory the device should be capable of providing adequate cardiac output without the coadministration of high dose vasopressors; however, the patient population requiring such significant support often prohibits the de-escalation of chemical therapy.
Complications
- Most common: vascular (bleeding, hematoma, vessel perforation, dissected artery)
- These complications have been shown to be minimized through vascular access techniques that include the use of fluoroscopy, ultrasound, micropuncture, angiography, and vascular closure devices.15
- Hematologic (thrombosis and hemolysis)
- Thrombosis in this patient population should be relatively rare. Most patients with an Impella® are on systemic anticoagulation, and the device’s purge solution also contains anticoagulants. In some circumstances, patients may be on a combination of anticoagulation and antiplatelets. The intent of the medication is to decrease the risk of thrombus formation along the catheter or in the body of the Impella®
- Hemolysis is more common in patients with Impella® due to the nature of a rotary pump accelerating blood through a narrow channel. This can be monitored with labs including LDH, haptoglobin, plasma free Hgb, d-dimer, and others. In patients with Foley catheters in place, color of urine can also be monitored for signs of hemolysis.14
- Arrhythmia
- The presence of an Impella® predisposes patients to cardiac arrhythmia.
- Device positioning
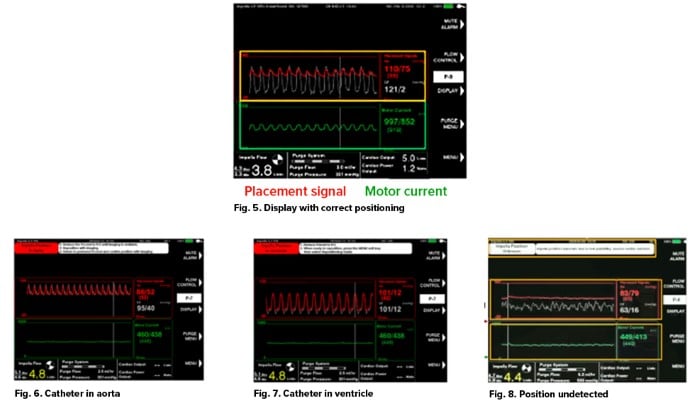
- The appropriate positioning of an Impella® can be seen on ultrasound. When in the appropriate position, the Impella® interface will demonstrate an aortic waveform in red, a left ventricular waveform in white, and a motor pump pulsatility waveform in green (see Fig 5).16
- Two major malpositioning events may occur in patients with Impella® pumps: The catheter either may be displaced completely into the ventricle or be completely within the aorta.
- Impella® displacement into the aorta (See Fig. 6)16
- In this scenario, the optical sensor of the pump will generate an aortic waveform; however, the LV waveform will also take on this morphology. Simultaneously, the operator likely will notice the lack of motor pump pulsatility.
- Impella® displacement into the left ventricle (see Fig. 7)16
- In this scenario, the optical sensor for the pump will have descended into the LV. Therefore, both the aortic and ventricular waveform will take on an LV waveform. Simultaneously, the operator likely will notice the lack of motor pump pulsatility.
- Impella® Displacement with unknown location (see Fig. 8)16
- The placement signal will still be pulsatile but will have dampened amplitude, and motor current will be flat or dampened.
- Impella® displacement into the aorta (See Fig. 6)16
- Suction event
- A suction event occurs when the device accidentally suctions up against native heart tissue at the level of the inlet (drainage) port, leading to restriction or complete obstruction of blood flow. Suction events can occur for many reasons. Anything that decreases the initial LV volume has potential to cause a suction event. Consider a hypovolemic patient with an Impella® running at P9: this could lead to suction events because the LV is not being adequately filled to support the amount of forward flow the pump is trying to produce. Similarly, RV failure can cause suction events due to inability of the RV to provide appropriate preload for the LV.16 (See Fig. 9)
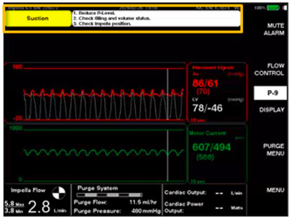 Fig. 9. Standard device reading during suction event
Fig. 9. Standard device reading during suction event
-
- Identification of suction events is crucial to good patient care. Prior to the device alarming, you may see lower-than-expected flows, decreased blood pressure, or a reduced mean motor current when graphed over a 5-minute period.
- Coding patient with Impella®
- Unlike a patient on VA-ECMO, if your patient with an Impella® suffers cardiac arrest, they should be resuscitated per standard ACLS protocol. Reduce the pump to flow P2 and conduct the code as normal. There is no contraindication to defibrillation in these patients. If ROSC is achieved, the flow on the Impella® may be titrated up as is appropriate for the patient's hemodynamics. An echocardiogram should also be performed following ROSC to ensure there was no migration of the catheter during compressions.17
Troubleshooting
- Hematoma or bleeding at insertion site: Management is somewhat dependent on which Impella® device is used and where it is inserted.
- Hemolysis/thrombosis: If CVP or PCWP are <10mmhg, consider adding more volume to improve preload and decrease shear forces. If preload is adequate and patient’s hemodynamics are improved as well, consider decreasing flow on the machine to further decrease shear forces.17 As mentioned above in regard to suction events, any suction event is also going to increase your shear forces and therefore worsen hemolysis. If problems arise with the systemic anticoagulation itself such as HIT, systemic anticoagulation should be switched to a non-heparin based alternative such as argatroban.18
- Device malposition: This may initially be recognized based on machine alarms but also through visualization of the placement signal and motor current waveform. Confirm with echo, and adjust at bedside for most of the devices, with repeat imaging following repositioning.
- Suction event: In order to address the suction event, we must understand its etiology. If the suction is occurring due to inadequate LV volume, you must assess the patient's hemodynamics. If they have been improving and the patient is on a high level of support such as P7-9, consider dropping the flow and reassessing pressure. This will of course decrease the forward flow, which will inherently increase the LV volume – reducing further suction events.17 If the patient requires the current level of support or even more forward flow, consider giving volume. If your CVP or PCWP are less than 10, you can consider administration of volume to see if this will improve the suctioning.17 Note: There is a possibility of concomitant RV failure. This can be measured and assessed in many ways, depending on which invasive hemodynamic monitoring is being used. In these patients, consider in conjunction with cardiology or CT surgery the placement of an RV pump (Impella® RP). Similar to left-sided pumps, the Impella® RP will offload the work of the right ventricle and improve the LV filling due to increased forward flow from the RV. If the suction event is occurring at the level of the aorta, assess what else your patient is currently receiving for hemodynamic support.
TAKE-HOME POINTS
- The Impella® is a catheter-based left ventricular assist device that uses axial flow to provide mechanical circulatory support for patients experiencing a multitude of different pathologies resulting in cardiogenic shock. It is preload-dependent and afterload-sensitive, which differentiates it from other assist devices such as the IABP, which are rhythm dependent.
- The Impella® is useful in numerous clinical scenarios, among which are patients undergoing high-risk PCI, patients experiencing cardiogenic shock from an acute MI or other underlying pathology, and patients experiencing persistent malignant arrhythmias.
- There are various Impella® devices available based on the amount of forward flow your patient requires, ranging from 2.5L of CO to full support at 5.5L seen with the Impella 5.5®. There also exists an Impella specifically for patients experiencing RV failure (Impella RP®).
- As with any device or procedure, there are complications to be aware of in your patients receiving mechanical circulatory support. It is incumbent upon the physician to appropriately manage these complications.
- Any patient who experiences cardiac arrest should be resuscitated without reservation, just as you would a patient without an Impella® The only thing to remember is to drop the flow to level P-2, and if ROSC is achieved, obtain a stat echo to ensure appropriate cannula position.
References
- Glazier JJ, Kaki A. The Impella Device: Historical Background, Clinical Applications and Future Directions. Int J Angiol. 2019;28(2):118-123.
- Burzotta F, Trani C, Doshi SN, et al. Impella ventricular support in clinical practice: Collaborative viewpoint from a European expert user group. Int J Cardiol. 2015;201:684-91.
- Lo N, Magnus Ohman E. Mechanical Circulatory Support in ST-Elevation Myocardial Infarction. In: Watson TJ, Ong PJL, Tcheng JE, eds. Primary Angioplasty: A Practical Guide [Internet]. Singapore: Springer; 2018.
- Alba AC, Rao V, Ivanov J, Ross HJ, Delgado DH. Usefulness of the INTERMACS scale to predict outcomes after mechanical assist device implantation. J Heart Lung Transplant. 2009;28(8):827-33.
- Thiele H, Zeymer U, Neumann FJ, et al., for the IABP-SHOCK II Trial Investigators. Intraaortic Balloon Support for Myocardial Infarction with Cardiogenic Shock. N Engl J Med. 2012;367(14):1287-1296.
- O'Neill WW, Schreiber T, Wohns DH, et al. The current use of Impella 2.5 in acute myocardial infarction complicated by cardiogenic shock: results from the USpella Registry. J Interv Cardiol. 2014;27(1):1-11.
- Møller JE, Engstrøm T, Jensen LO, et al., for the DanGer Shock Investigators. Microaxial Flow Pump or Standard Care in Infarct-Related Cardiogenic Shock. N Engl J Med. 2024;390:1382-1393.
- O'Neill WW, Kleiman NS, Moses J, et al. A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: the PROTECT II study. Circulation. 2012;126(14):1717-27.
- Perera D, Stables R, Thomas M, et al. Elective intra-aortic balloon counterpulsation during high-risk percutaneous coronary intervention: a randomized controlled trial. 2010;304(08):867–874.
- Reddy YM, Chinitz L, Mansour M, et al. Percutaneous left ventricular assist devices in ventricular tachycardia ablation: multicenter experience. Circ Arrhythm Electrophysiol. 2014;7(02):244–250.
- Rios L, Alsaad A, Minga I, et al. Impella Bridge To Surgery For Severe Aortic And Mitral Regurgitation. J Am Coll Cardiol. 2021;77 (18_Supplement_1) 2898.
- Miller MA, Dukkipati SR, Chinitz JS, et al. Percutaneous hemodynamic support with Impella 2.5 during scar-related ventricular tachycardia ablation (PERMIT 1). Circ Arrhythm Electrophysiol. 2013;6(01):151–159.
- Yeo I, Wong SC, Mack CA, et al. Feasibility and Safety of Impella-Assisted High-Risk PCI Before TAVR in Patients With Severe Aortic Stenosis. J Soc Cardiovasc Angiogr Interv. 2023;2(5):101061.
- Zein R, Patel C, Mercado-Alamo A, Schreiber T, Kaki A. A Review of the Impella Devices. Interv Cardiol. 2022;17:e05.
- Sandoval Y, Burke MN, Lobo AS, et al. Contemporary arterial access in the cardiac catheterization laboratory. JACC Cardiovasc Interv. 2017;10:2233–41.
- ABCs of Impella. Undated.
- Balthazar T, Vandenbriele C. Part 9: Impella Troubleshooting and Resuscitation. In: Handbook on Mechanical Circulatory Support. Vranckx P, ed. European Society of Cardiology. 2022;115-123.
- Hohlfelder B, Militello MA, Tong MZ, et al. Anticoagulation with temporary Impella device in patients with heparin-induced thrombocytopenia: a case series. Int J Artif Organs. 2021;44:367-370.