It was 9 a.m. on my first day in the cardiothoracic intensive care unit (CT-ICU), a unit I had never set foot in before. As rounds began, we stopped at the bedside of a patient in his mid-50s with a history of biventricular heart failure. The attending casually mentioned he was on left atrial veno-arterial extracorporeal membrane oxygenation (LAVA-ECMO).
Now, I have read about veno-venous ECMO (V-V ECMO), veno-arterial ECMO (V-A ECMO), and their various cannulation strategies. But LAVA-ECMO? That one had not made it into my residency syllabus. Before I could discreetly reach for my phone to consult Dr. Google, the attending turned and asked, "Do you know what LAVA-ECMO is and how it's different?"
I admitted I did not.
But instead of being grilled for my ignorance, she took a moment to walk me through it and illustrated how LAVA-ECMO works, why it is used, and what makes it unique. That 5-minute teaching moment stuck with me.
This article pays that moment forward. We will break down LAVA-ECMO in a clear, concise, and high-yield way, so the next time you are in the CT-ICU (or hear the term during a code or sign-out), you will not only understand what is happening physiologically, but you will be ready to explain it to others as well.
ECMO is an advanced form of mechanical circulatory support (MCS) designed to provide temporary cardiopulmonary assistance in patients experiencing severe cardiac and/or respiratory failure that is refractory to conventional management. ECMO involves withdrawing blood from the venous system, directing it through an external membrane oxygenator for gas exchange, and subsequently returning the oxygenated blood to the patient's circulation. The specific configuration of ECMO depends on the site of blood return. In V-V ECMO, blood bypasses the lungs and is returned to the venous system, thereby supporting primarily pulmonary function, with potential indirect support for the right heart. In contrast, V-A ECMO returns blood to the arterial system, offering both cardiac and respiratory support by bypassing the heart and lungs. V-V and V-A ECMO represent the 2 principal modalities of ECMO, selected based on the patient’s underlying pathology. It is critical to understand that ECMO does not serve as a curative treatment; rather, it serves as a bridge to recovery, transplant, durable mechanical support, or hemodynamic stabilization while the affected organs recover.
Over the past 3 decades, the global utilization of ECMO has increased significantly. Data from the Extracorporeal Life Support Organization (ELSO) registry indicate that more than 245,000 cases have been recorded to date, with the number of participating ECMO centers expanding nearly sevenfold since 1990.¹ Despite its lifesaving potential, ECMO remains a complex and resource-intensive intervention, and its optimal integration into broader health care systems continues to prompt important clinical and ethical discussions.
For those looking to build a foundational understanding of ECMO, EMRA’s EM Resident Magazine offers an excellent primer by Lucas et al., titled "Critical Care ECMO Series: Introduction to ECMO." That series provides an in-depth exploration of ECMO’s core principles, modes of operation, clinical indications, and future directions. In this article, however, we will dive directly into a more advanced and specific V-A ECMO configuration: LAVA-ECMO.
A major limitation of V-A ECMO, particularly in the peripheral cannulation configuration, lies in its delivery of non-physiologic continuous retrograde blood flow into the aorta. This results in a significant increase in left ventricular (LV) afterload, placing additional strain on an already failing LV. When the LV is unable to generate sufficient pressure to overcome the elevated afterload, this can lead to progressive LV distension, impaired coronary perfusion, and stasis of blood within the left heart and aortic root. These conditions collectively increase the risk of thrombus formation and exacerbate pulmonary congestion.3,4
In response to these adverse hemodynamic effects, several strategies have been introduced to unload or vent the LV in order to reduce LV volume and thereby pressure. LAVA-ECMO has emerged as a hybrid approach that combines the benefits of conventional V-A ECMO with targeted LV unloading or venting strategies.
Discussion
V-A ECMO, while providing robust systemic perfusion in cardiogenic shock, can significantly increase LV afterload. This retrograde aortic flow elevates myocardial oxygen consumption, LV end-diastolic pressure (LVEDP), and pulmonary venous congestion, potentially exacerbating pulmonary edema and impairing myocardial recovery.3,4 To address these hemodynamic drawbacks, several adjunctive strategies have been employed, including inotropic support, intra-aortic balloon pumps (IABP), percutaneous ventricular assist devices such as the Impella, and surgical venting techniques like atrial septostomy.3,5
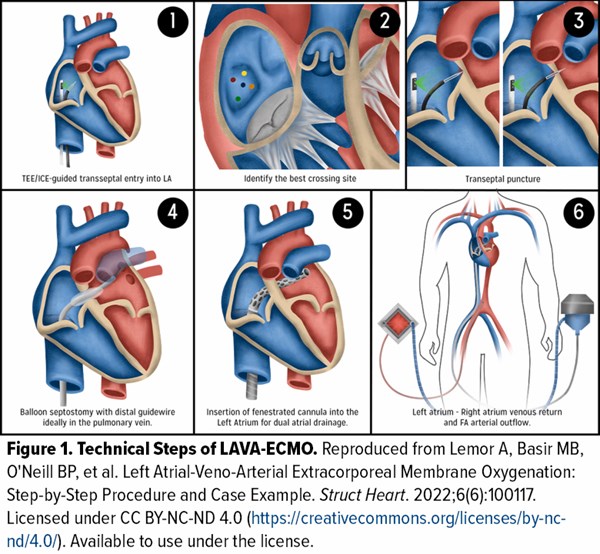
LAVA-ECMO represents an evolution of standard VA-ECMO, designed specifically to facilitate LV decompression through trans-septal venous drainage. In this approach, a fenestrated cannula is inserted across the atrial septum into the left atrium (LA), allowing for simultaneous right atrial (RA) and LA drainage into the ECMO circuit (technical steps illustrated in Figure 1).⁶ This configuration aims to reduce pulmonary capillary wedge pressure (PCWP) and LVEDP by directly unloading the LV, thereby mitigating pulmonary congestion and myocardial strain. Importantly, this technique accomplishes these goals without requiring additional large-bore access or insertion of a second mechanical device.6,7 Beyond hemodynamic optimization, LAVA-ECMO may also reduce the risk of intracardiac thrombus formation due to decreased stasis in the LV and may enhance myocardial recovery.
In patients with cardiogenic shock (CS) secondary to valvular heart disease (VHD), LAVA-ECMO provides distinct physiologic advantages by bypassing valvular lesions affecting both sides of the heart.⁸ Compared to traditional V-A ECMO, LAVA-ECMO offers more targeted support by directly draining the LA, thereby reducing LV pressure and relieving pulmonary congestion. This is particularly beneficial in conditions such as severe aortic regurgitation (AR) or stenosis (AS), mitral regurgitation (MR) and post-infarction ventricular septal defects (VSD), where conventional V-A ECMO may increase afterload and exacerbate LV strain. In a retrospective study of 18 patients with VHD, LAVA-ECMO was associated with significant reductions in pulmonary artery (PA) systolic pressure, PCWP, LVEDP, and RA pressure.8 Furthermore, LAVA-ECMO lowers afterload and mitigates pathologic shunting. Unlike V-A ECMO, which can elevate systemic pressures and worsen regurgitant or left-to-right intracardiac shunts, LAVA-ECMO improves overall hemodynamics by decreasing left-sided filling pressures. In addition, LAVA-ECMO can achieve both circulatory support and LV unloading through a single multistage venous cannula inserted transeptally, obviating the need for additional devices or arterial cannulation typically required with VA-ECMO and thereby reducing complications such as limb ischemia.9
Patient selection for LAVA-ECMO differs substantially from that of conventional V-A ECMO due to its distinct hemodynamic benefits and technical considerations. V-A ECMO is commonly indicated for a broad range of CS etiologies, including acute myocardial infarction, myocarditis, post-cardiotomy syndrome, and sepsis-induced myocardial dysfunction, and can often be initiated rapidly via peripheral cannulation without the need for intracardiac access.10 However, a known limitation of V-A ECMO is its tendency to increase afterload, which may lead to LV distension and worsening pulmonary edema, particularly in patients with severe AR, MR, AS, or post-infarction VSD.11,12
LAVA-ECMO, by contrast, is particularly well-suited for patients with CS accompanied by elevated LA or LV filling pressures, especially in the context of VHD or structural cardiac lesions. By draining blood directly from the LA, LAVA-ECMO reduces both preload and afterload while avoiding the need for additional LV venting strategies, making it advantageous in clinical settings where conventional VA-ECMO may aggravate regurgitant lesions or intracardiac shunting.13,14 The approach requires transseptal access and high-quality intracardiac imaging, making it most appropriate for patients with stable enough hemodynamics and suitable atrial anatomy to safely undergo the procedure. It is generally contraindicated in patients with isolated RV failure, mechanical mitral valves, or in situations requiring immediate deployment of circulatory support.15
While both modalities offer systemic perfusion support, LAVA-ECMO provides more physiologically targeted unloading in select patients with VHD-related shock, assuming technical feasibility and anatomical suitability.13,16
LAVA-ECMO is contraindicated in patients with severe RV failure without left-sided overload, as it primarily unloads the LA and indirectly the LV, leaving isolated RV dysfunction unaddressed. The presence of a mechanical mitral valve poses a significant risk due to potential obstruction or damage during transseptal cannulation. Additionally, anatomical barriers such as large atrial septal defects or thrombus, severe coagulopathy, active bleeding, and hemodynamic instability that preclude safe transseptal puncture are absolute contraindications. Relative contraindications include significant right-sided heart pathology requiring separate RV support, severe pulmonary hypertension, prior atrial septal interventions that complicate cannulation, limited imaging capabilities for transseptal guidance, and peripheral vascular disease affecting arterial access for VA-ECMO return cannulation. Furthermore, LAVA-ECMO is generally not advised in patients with end-stage multi-organ dysfunction where recovery or bridging is not feasible. These factors must be carefully considered to optimize patient selection and procedural safety.
The clinical utility of LAVA-ECMO is supported by a growing number of case reports and observational studies. In a 2021 case report, Singh-Kucukarslan et al.17 described a 59-year-old male with inferior ST-elevation myocardial infarction (STEMI) and CS unresponsive to Impella and high-dose vasopressors. Escalation to LAVA-ECMO significantly improved hemodynamics, with mean PA pressures falling from 38 mm Hg to 10 mm Hg, and cardiac index rising from 1.8 to 5.1 L/min/m². Despite failed LAVA-ECMO weaning attempts, the patient remained stable on support and underwent successful orthotopic heart transplantation 11 days later.
Similarly, Jiménez-Rodríguez et al.18 in 2023 reported a case of a 53-year-old man with CS secondary to post-infarction VSD. Initial support with IABP and V-A ECMO proved insufficient. Transitioning to LAVA-ECMO led to immediate hemodynamic stabilization, with reductions in PCWP and pulmonary (Qp)/systemic (Qs) flow ratio from 9.81 to 2.15. Although the patient later developed an ischemic and then hemorrhagic stroke, no evidence of thrombus was found on transesophageal echocardiography. Most recently, a 2025 single-center retrospective analysis by Giustino et al.⁸ evaluated outcomes in patients with valvular CS managed with LAVA-ECMO. Among 18 patients treated between 2018 and 2023, the intervention was associated with significant reductions in RA pressure (–8 mm Hg), mean PA systolic pressure (–18.5 mm Hg), PCWP (–14.5 mm Hg), and LVEDP (–20 mm Hg), all with statistically significant confidence intervals. The intervention showed a 69.1% bridge-to-procedure rate and 44.4% survival to hospital discharge, with no procedural complications reported from trans-septal access.
Collectively, these cases underscore the hemodynamic benefits and feasibility of LAVA-ECMO as an advanced unloading strategy with venting capabilities. However, broader adoption will require prospective studies to validate its safety and efficacy in diverse patient populations. As such, consideration for LAVA-ECMO should be reserved for high-volume tertiary care centers with expertise in advanced ECMO management and trans-septal interventions.
Take-Home Points
- Understanding ECMO basics is essential, even in the ED: While V-V and V-A ECMO may not be initiated in the ED always, understanding their underlying physiology is critical during handoffs, transfers, or codes.
- Peripheral V-A ECMO increases afterload: Retrograde flow from peripheral VA-ECMO increases afterload on the LV, which may exacerbate LV distension, elevate PCWPs, and lead to pulmonary edema or thrombus formation. Be alerted to worsening oxygenation, increased pulmonary pressures, or persistently low LV output, even if the ECMO circuit appears to be functioning.
- LAVA-ECMO offers dual atrial decompression with V-A ECMO support: LAVA-ECMO integrates a trans-septal drainage cannula into the ECMO circuit, enabling direct unloading of the LA alongside RA drainage. This configuration reduces afterload and facilitates LV decompression without the need for an additional device, such as an Impella.
- LAVA-ECMO offers targeted circulatory support in VHD related cardiogenic shock by directly unloading the LA, reducing LV pressure and pulmonary congestion. It is especially advantageous in conditions like severe AR, MR, AS, and post-infarction VSD, where VA-ECMO may worsen hemodynamics. By avoiding additional devices and arterial access, LAVA-ECMO simplifies management while minimizing complications.
- LAVA-ECMO is unsuitable for patients with isolated RV failure, mechanical MV, or anatomical and hemodynamic conditions that preclude safe transseptal access. Relative contraindications include severe PAH, prior septal interventions, or limited imaging access. Careful patient selection is essential to ensure procedural success and minimize risk.
- Ask early and learn often: Detailed knowledge of ECMO and its various configurations, early in training, is not expected. However, asking questions (“Is this a LAVA-ECMO configuration?”) shows initiative and creates meaningful opportunities to learn about advanced circulatory support and hemodynamics from your ICU team.
References
- Extracorporeal Life Support Organization (ELSO). ELSO Registry Report. Published July 2023. Accessed July 20, 2025. https://www.elso.org/Registry/Statistics.aspx
- Williams B, Bernstein W. Review of venoarterial extracorporeal membrane oxygenation and development of intracardiac thrombosis in adult cardiothoracic patients. J Extra Corpor Technol. 2016;48(4):162-167.
- Cevasco M, Takayama H, Ando M, et al. Left ventricular distension and venting strategies for patients on venoarterial extracorporeal membrane oxygenation. J Thorac Dis. 2019;11(4):1676-1683. doi:10.21037/jtd.2019.03.29.
- Cheng R, Hachamovitch R, Kittleson M, et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1,866 adult patients. Ann Thorac Surg. 2014;97(2):610-616. doi:10.1016/j.athoracsur.2013.09.008.
- Schrage B, Becher PM, Bernhardt A, et al. Left ventricular unloading is associated with lower mortality in patients with cardiogenic shock treated with venoarterial extracorporeal membrane oxygenation: results from an international, multicenter cohort study. Circulation. 2020;142(22):2095-2106. doi:10.1161/CIRCULATIONAHA.120.048792.
- Lemor A, Basir MB, O'Neill BP, et al. Left atrial–venoarterial extracorporeal membrane oxygenation: step-by-step procedure and case example. Struct Heart. 2022;6(6):100117. doi:10.1016/j.shj.2022.100117.
- Villablanca PA, Al-Darzi W, Boshara A, et al. Left atrial venoarterial extracorporeal membrane oxygenation for patients in cardiogenic shock and acute aortic regurgitation. JACC Cardiovasc Interv. 2022;15(20):2112-2114. doi:10.1016/j.jcin.2022.08.015.
- Giustino G, Fadel RA, Jabri A, et al. Left atrial veno-arterial extracorporeal membrane oxygenation in valvular cardiogenic shock. J Soc Cardiovasc Angiogr Interv. 2025;4(5):102615. doi:10.1016/j.jscai.2025.102615.
- Jones CR. Left atrial decompression during veno-arterial extracorporeal membrane oxygenation using a single multi-stage drainage cannula with a transseptal approach: Clinical significance and medical management of LAVA ECMO. World J Biomed Pharm Sci. 2024;20(2):0889. doi:10.30574/wjbphs.2024.20.2.0889.
- Aissaoui N, Carrié D, Mastroianni C, et al. Venoarterial extracorporeal membrane oxygenation for cardiogenic shock: indications and outcomes. Eur Heart J. 2020;41(23):2111-2119. doi:10.1093/eurheartj/ehaa070.
- Genuardi MV, Vervoort D, Rosati CM, et al. Limitations of venoarterial extracorporeal membrane oxygenation in structural heart disease: a review of pathophysiology and management. JACC Case Rep. 2022;4(7):605-613. doi:10.1016/j.jaccas.2022.03.004.
- Manganas C, Panos A, Papadopoulos C, et al. Impact of venoarterial extracorporeal membrane oxygenation on afterload and mitral regurgitation severity in patients with ventricular septal defects. ASAIO J. 2023;69(1):47-53. doi:10.1097/MAT.0000000000001658.
- Jones CR. Left atrial decompression during venoarterial extracorporeal membrane oxygenation using a single multistage drainage cannula with a transseptal approach: clinical significance and medical management of LAVA ECMO. World J Biomed Pharm Sci. 2024;20(2):0889. doi:10.30574/wjbphs.2024.20.2.0889.
- Gannon MP, Smith JF, Patel N, et al. Novel approaches to left atrial drainage in extracorporeal membrane oxygenation for valvular heart disease. J Card Surg. 2023;38(5):874-881. doi:10.1111/jocs.16850.
- Raman J, Chen S, Lee D, et al. Patient selection and procedural considerations for left atrial venoarterial extracorporeal membrane oxygenation: early clinical experience. J Thorac Cardiovasc Surg. 2022;163(3):1082-1089. doi:10.1016/j.jtcvs.2021.05.067.
- Basir MB, Patel SM, Shekar K, et al. Outcomes of left atrial venoarterial extracorporeal membrane oxygenation in structural heart disease: a multicenter cohort study. ASAIO J. 2024;70(2):122-130. doi:10.1097/MAT.0000000000001743.
- Singh-Kucukarslan G, Raad M, Al-Darzi W, et al. Hemodynamic Effects of Left-Atrial Venous Arterial Extra-Corporeal Membrane Oxygenation (LAVA-ECMO). ASAIO J. 2022;68(9):e148-e151. doi:10.1097/MAT.0000000000001628.
- Jiménez-Rodríguez GM, Rojas-Velasco G, Arias-Sánchez EA, et al. Left atrial veno-arterial extracorporeal membrane oxygenation in post-myocardial infarction ventricular septal defect with cardiogenic shock: a case report. Eur Heart J Case Rep. 2023;7(8):ytad393. doi:10.1093/ehjcr/ytad393.