Extracorporeal membrane oxygenation (ECMO) allows for temporary life support in cardiopulmonary failure refractory to conventional medical treatment. In this first of our EMRA Critical Care ECMO Series, we explain the history and rise of this novel treatment.
Introduction
"During that long night, helplessly watching the patient struggle for life, the idea naturally occurred to me that if it were possible to remove continuously some of the blue blood from the patient's distended veins, put oxygen into that blood and allow carbon dioxide to escape from it, and then inject continuously the now red blood back into the patient's arteries, we might have been able to save her life."
- Dr. John Gibbon, recounting the loss of a young female from a fatal pulmonary embolism (PE), 19311
Extracorporeal membrane oxygenation (ECMO) allows for temporary life support in cardiopulmonary failure refractory to conventional medical treatment. It is a technology that has been in conceptual development for centuries, but has since come to practice over the past 50 years. In simple terms, ECMO allows one to partially bypass the lungs, the heart, or both. This allows for a wide range of potential applications including cardiac arrest, refractory respiratory failure, transplant medicine, and much more. It serves as a temporary bridge for the critically ill patient with a potentially reversible underlying medical condition. While this technology can, and has, provided miracles for numerous patients throughout the world, it is extremely resource-intensive, and its role in the larger health system is still debated.
ECMO has gained considerable popularity over the past 30 years. There are more than 200,000 ECMO cases reported in the Extracorporeal Life support Organization (ELSO) registry, and almost seven-fold increase in the number of centers with ECMO since 1990.2 There are a number of ECMO applications relevant to emergency medicine. In some locations, emergency medicine physicians perform ECMO cannulation, and emergency medicine intensivists are often members and leaders of ECMO teams. Given its capabilities and increasing presence in our health care system, it is important that all learners, from medical students to attending physicians, become familiar with the technology as it expands to more ICUs and EDs across the world.
We aim to present the core principles of ECMO through a series of deep-dive articles. These will include the basics of ECMO technology, operational modes, clinical indications, and future applications.
A Brief History of ECMO
Although ECMO was first successfully performed in the late 1900s, the idea was not novel to the 20th century. Once physician William Harvey recognized and published in 1628 that blood was pumped through a system of arteries and veins, the conceptual race toward ECMO had begun. Prior to this, medicine widely believed in the ancient Greek philosophy proposed by Galen (AD 129) who stated that blood was carried in two separate systems and terminated in the arteries and veins separately.3 Additional scientists of the 17th century including scientist and philosopher Robert Hooke proposed that exposing blood to fresh air, not simply the motion of lungs, might suffice for life.4
Toward the 19th century there were various experiments that extended the concept of oxygenating blood and the eventual development of oxygenators. In 1849, physiologist Julien-Jean Cesar la Gallois failed at perfusing decapitated rabbits by injecting arterial blood, but later successfully perfused an isolated kidney. In 1882, scientist Von Schroeder bubbled oxygen through venous blood and constructed the first bubble oxygenator. This was unfortunately limited by foaming. An additional type of oxygenator was developed by Max von Frey and Max Gruber in 1882. They constructed a rotating film oxygenator rotating at 30 RPM.4 They further developed their device by adding heating and cooling chambers, valves, and syringe pumps to maintain blood flow. These cylinder oxygenators were later improved and used in initial cardiopulmonary bypass surgeries.5 The early part of the 1900s gave way to another discovery essential for ECMO use today. Medical student Jay McClean discovered a phosphatide extracted from canine heart muscle that prevents blood clotting. This would later be isolated from liver tissue and given the name heparin.4 Further characterization of ABO blood types and transfusion reactions also improved efforts. Coagulation management remains a challenge today in ECMO use and would not have been successfully implemented without these advances in hematology.
In the 20th century we began to have the first working applications of ECMO. In 1931, Dr. John Gibbon Jr. had a devastating case of a young woman with a PE. This kickstarted an increased effort to develop ECMO that could be used for medical procedures. At this time, three main types of oxygenators were in development: rotating disc oxygenators, improved bubble oxygenators, and film oxygenators. Dr. Gibbon worked for decades to optimize his film oxygenator and was successful in performing cardiopulmonary bypass on cats in 1939. His work expanded and his team performed the first successful complete bypass for the closure of an atrial septal defect in an 18-year-old woman in 1953.1 This was a great achievement; however, bypass was unable to be achieved for more than a few hours as the blood was damaged when directly contacted with air and by components of the pump. Over the next decade, oxygenator technology progressed and in 1963, Dr. Theodor Klobow created a new membrane oxygenator ready for commercial use. This differed from prior versions in that there was no direct blood-air contact as blood was run through a specialized tubing wrapped by fibers of oxygen.7

Figure 1: The Gibbon heart-lung machine used for ASD repair in 1953 (Credit: Richard W. Strauss, National Museum of American History)
Expansion of oxygenator technology allowed for advances of cardiopulmonary bypass procedures throughout the 1960s. Heart transplants and other complex cardiopulmonary procedures became possible with these early machines. In 1972, the first successful use of ECMO in an acute respiratory distress syndrome (ARDS) patient post-trauma was reported with an ECMO duration of 75 hours (Figure 2).8 Dr. Robert Bartlett further expanded the technology to the world of neonatal respiratory failure and reported a 90% success rate for most cases of neonatal respiratory failure by 1984.9 He was and continues to be a fierce advocate for ECMO technology and created the Extracorporeal Life Support Organization (ELSO). By many, he is referred to as the Father of ECMO, as his success and advocacy has inspired others to expand and research the technology. Hollow fiber oxygenators were developed for ECMO in the 1970s and modern versions of this technology are used today. Post 1990, trials and more reports demonstrated the potential success and ECMO expanded throughout the world.
Today, ECMO is used in over 50 countries with greater than 200,000 cases reported per the ELSO registry.2 The early conceptual ideas, engineering discoveries, and medical advances have inspired a movement that has saved thousands of lives.
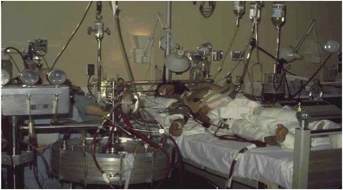
Figure 2. First patient treated on V-V ECMO
Many other developments were essential to build ECMO into what we see today. These are just a few examples, but do not capture nearly all of the contributions. ECMO technology continues to be optimized and expanded to further applications through clinicians and scientists working together across the world.
Indications
In general, ECMO is reserved for critically ill patients with reversible pathologies. ECMO is considered at 50% mortality risk and indicated at 80% risk.10 Specific indications for ECMO cannulation vary by center. Several scoring systems have been investigated to predict the best candidates for ECMO; however, a universal scoring system has yet to be adopted. In general, ECMO is used for conditions with one or more of the following characteristics:
- Acute severe cardiac or respiratory failure
- A high predicted risk of mortality
- Not responsive to optimal conventional therapy
- Potential reversibility or healing with time
- As a bridge to definitive treatment such as device implantation or solid organ transplant
V-V ECMO
- P:F ratio < 50 mmHG for > 3 hours despite
- Optimization of mechanical ventilation (FiO2 ≥ 80%, PEEP ≥ 10 cm H2O, Vt 6 cc/kg) and
- Use of adjunctive therapies for ARDS (prone positioning, paralysis, recruitment maneuvers, iNO)
- P:F ratio < 80 mmHG for > 6 hours despite above criteria
- Arterial blood pH < 7.25 with PaCO2 ≥ 60mmHg for > 6 hours with
- RR increased to 35 breaths / minute and
- Vent settings adjusted to keep plateau pressure ≤ 32 mmHg
Example of V-V applications include: ARDS, pulmonary contusion, inhalation injuries (including drowning, smoke, gastric contents), status asthmaticus, graft failure post-lung transplant, bridge to lung transplant
V-A ECMO
- Refractory cardiogenic shock
- Failing ≥ 2 inotropes or a mechanical device
- Biventricular failure
- Refractory cardiac arrest
- No ROSC within 10 minutes
- Suspected reversible etiology
- Witnessed event with immediate CPR
- Time from arrest to ECMO flow < 60 minutes
- “ECMO-CPR or eCPR”
- Bridge to recovery, device or transplant
Examples of V-A applications include: ischemic cardiogenic shock, massive pulmonary embolism, bridge to definitive cardiac support (corrective surgical procedure, ventricular assist device, or heart transplant), acute decompensated cardiomyopathy, toxic emergencies, acute fulminant myocarditis, hypothermia, acute graft failure post-heart transplant, postcardiotomy cardiogenic shock, sepsis with profound cardiac depression, refractory arrhythmias
Contraindications
Contraindications are often relative and depend on the specific ECMO center.10
General
- Advanced age, typically older than 75 but depends on the ECMO indication and center
- Severe neurologic dysfunction (relative contraindication)
- Significant comorbid disease
- Metastatic cancer, advanced cirrhosis, end-stage kidney disease
- Non-reversible illness or illness without a bridge to device or transplant
- Contraindication to anticoagulation (for VA ECMO)
- Morbid obesity or extreme body habitus that would make cannulation impossible
V-V specific
- Chronic severe pulmonary hypertension
- Prolonged mechanical ventilation (> 7 days) on high pressures and high FiO2
- Severe myocardial depression or advanced shock
V-A specific
- Unwitnessed cardiac arrest or CPR > 60 minutes prior to commencement of ECMO
- Aortic dissection
- Significant aortic valve incompetence
Basic ECMO Configurations
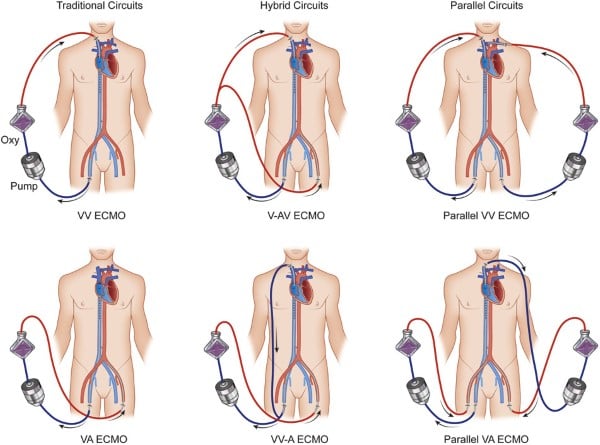
The two basic ECMO configurations are described below. The next articles in our series will go in-depth into the details of the circuits, the components, operation, and different variations.
V-V ECMO
V-V ECMO is used for respiratory failure refractory to mechanical ventilation. It only provides gas exchange and does not provide any component of circulatory support. In V-V ECMO, a centrifugal pump generates negative pressure via the “drainage” cannula and then pumps blood across a membrane lung or oxygenator, which adds oxygen and removes carbon dioxide. Oxygenated blood is returned to the body via the “return” cannula, typically positioned in a central vein or the right atrium, providing no circulatory support and adding the artificial lung in series with the native lung. V-V ECMO allows for the removal of carbon dioxide and return of oxygenated blood. Several parameters affect oxygen delivery, carbon dioxide removal, and the flow of blood. Oxygenation is determined by the FdO2 and blood flow rate. FdO2 is the fractional delivered O2 and is typically set at 100% initially. Blood flow rate is primarily determined by the pump speed (measured in revolutions per minute, or RPMs). Increasing flow by increasing RPMs can improve oxygen delivery, though ECMO pumps are sensitive to preload and afterload, which are also determinants of blood flow. Factors affecting preload and afterload on V-V ECMO include volume status, systemic vascular resistance, cannula size, and pressures in any of the body compartments through which the ECMO cannula travels (eg, abdomen, pleural space, pericardial space). Carbon dioxide removal is determined by the sweep. A higher sweep gas flow will clear CO2 more rapidly.
Cannulation for V-V ECMO can be done by either a double-lumen cannula or two single-lumen cannulas. Double-lumen cannula are placed in the right internal jugular (IJ) vein, where blood is extracted from the vena cava and returned to the right atrium. Single-lumen cannula systems are typically placed in the common femoral vein for drainage and either right IJ or femoral vein for return.
V-A ECMO
V-A ECMO allows for both circulatory support and gas exchange. This is used in the setting of cardiogenic shock or combined cardiopulmonary failure to augment cardiac output. V-A ECMO pulls deoxygenated blood from the patient’s venous circulation via a large-bore cannula into a pump, then passes that blood into an oxygenator for gas exchange, and finally returns oxygenated blood into arterial circulation via another large-bore cannula. This circuit runs in parallel to the body's native circulation. This allows ECMO to support both heart and lung function. The location of arterial return depends on the configuration.
Cannulation can be central or peripheral. Central cannulation involves the great vessels and is typically done in the OR for short-term support. Most patients are post-surgical intervention (typically postcardiotomy), who despite vasopressor and inotropic support are unable to be weaned from cardiopulmonary bypass. It involves a venous drainage cannula in the right atrium with the return cannula being placed in the ascending aorta or subclavian artery. These cannulations are typically performed by cardiothoracic surgeons.
Peripheral cannulation is typically done outside of the OR for management of patients with refractory cardiogenic shock or who are in cardiac arrest. The venous drainage cannula is placed in the femoral vein and sits with the distal tip in the proximal IVC or right atrium and an arterial return cannula is most commonly placed in the common femoral artery. These vessels are accessible for ED, ICU, cardiology, and cardiothoracic physicians outside of the OR. Bi-femoral access is common for patients in cardiac arrest for extracorporeal cardiopulmonary resuscitation (eCPR).11
Important additional considerations for V-A ECMO are left ventricle (LV) unloading and distal limb perfusion. During V-A ECMO, the arterial outflow cannula can generate retrograde flow toward the aortic valve, thus increasing afterload and causing LV stress. This can start a downward spiral of decreased LV function and increased myocardial oxygen demand. This can also decrease aortic valve opening, stasis, and thrombus formation. There are several techniques that can be used to mitigate this afterload problem, or “vent” the left ventricle. These techniques include left atrial or left ventricular surgical management, placement of an intra-aortic balloon pump, or placement of an Impella. Limb ischemia is also common on VA ECMO, particularly when a femoral arterial return cannula is in place, which can obstruct flow to the distal part of the ipsilateral leg. Close monitoring is necessary. A smaller distal perfusion cannula is often inserted distal to the ECMO cannula in the superficial femoral or posterior tibial artery and connected to the ECMO circuit, diverting well-oxygenated blood down the affected leg.
Major circuit components in V-A ECMO are similar to V-V ECMO. The main difference is that the outflow cannula is placed into an artery, there is often a need for LV venting or afterload reduction, and distal perfusion catheters can be placed to improve blood flow to extremities.
Major circuit components for both V-V and V-A ECMO
- Pump = drain blood from patient and drive it through oxygenator
- 2 types: Roller or Centrifugal
- Blood flow monitor = measure rate of blood flow
- Oxygenator = site of gas exchange. Basic structure of hollow fiber membrane which is an anatomical equivalent of alveoli in the lungs
- Pressure monitor = placed at different parts of ECMO circuit to monitor integrity of circuit components
- Heat exchanger = warmed by external water bath. Can be used for cooling, ie, neuroprotection, fever control, etc.
- Gas blender or mixer = air and oxygen mixed to deliver desired FiO2
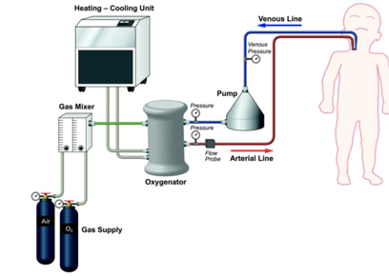
Figure 4: Basic ECMO Components13
Table 1: Complications
|
Complication |
Rate of complication |
|
Renal failure requiring renal replacement therapy |
52% |
|
Bacterial pneumonia |
32% |
|
Any bleeding |
33% |
|
Oxygenator dysfunction, requiring replacement |
29% |
|
Sepsis |
26% |
|
Hemolysis |
18% |
|
Livery dysfunction |
16% |
|
Leg ischemia |
10% |
|
Venous thrombosis |
10% |
|
CNS complications |
8% |
|
Gastrointestinal bleeding |
7% |
|
Aspiration pneumonia |
5% |
|
Disseminated intravascular coagulation |
5% |
Table 1: Rates of complications in V-A ECMO (table created from meta analysis)14
ECMO complications are common and expected, especially given how ill patients receiving this intervention are combined with the complexity of the circuit.
One of the most common complications is hemorrhage.15 Coagulopathy develops due to the shear stress of continuous blood flow and due to the patient’s blood in the circuitry activating the coagulation cascade. This leads to thrombocytopenia, factor XIII deficiency, acquired von Willebrand syndrome, and fibrinogen deficiency. The patient is also typically anticoagulated while on ECMO to avoid circuit thrombosis, especially on V-A ECMO. This leads to multiple sources of possible hemorrhage, including ECMO cannulation sites, intrathoracic, intraabdominal, intracerebral, and retroperitoneal regions. The distal perfusion catheter can also become dislodged, which can serve as a major source of bleeding into the leg compartment. Given the use of heparin, patients may also develop heparin induced thrombocytopenia.
ECMO also activates coagulation and inflammatory cascades that release prothrombotic substances in the body, thus increasing the risk for both arterial and venous thromboemboli.16 Common complications also include clot formation in the ECMO circuit. Clots in the oxygenator can lead to failure or need to replace portions of the ECMO circuit. When the circuit needs to be exchanged, patients experience a large inflammatory response, often resulting in the need for vasopressors in addition to ECMO support.
Other neurologic complications, outside of hemorrhage, are infarction and seizure. This risk is highest in neonates.
Multiple medical complications can also occur. Acute kidney injury is commonly seen and thought to be secondary to ischemic-reperfusion injury. Patients can often have difficult to manage hypertension, arrhythmias, oliguria, acute tubular necrosis, GI bleed, biliary calculi/hyperbilirubinemia. Metabolic complications due to electrolyte imbalance or blood sugar abnormalities can also occur. Infection rates are high on patients on ECMO given the large ECMO cannula and prolonged hospital course for these patients. Patients are commonly found to have bloodstream and surgical site infections. The causative organisms often include coagulase-negative Staph, Candida, and Pseudomonas.15 The role of prophylactic antibiotics is unclear.
V-A ECMO carries higher a higher risk of complication than V-V ECMO.16 Cannulation of the femoral artery carries risk of lower extremity ischemia due to clotting or obstruction of arterial flow from the ECMO cannula itself. Regardless of the site, it can lead to dissection or pseudoaneurysm. When the femoral artery is used, there is a risk of missing poor perfusion of the upper extremities and the brain if oxygen is preferentially delivered to the lower extremities and abdominal circulation, especially as the native heart recovers and pumps poorly oxygenated blood from the lungs. This phenomenon is called dual circulation, differential hypoxemia, or North South Syndrome. A right radial arterial line to measure upper extremity arterial blood gasses or a pulse oximeter on the right upper extremity are important tools to monitor for this phenomenon. It can lead to decreased cerebral oxygenation and anoxic brain injury. Patients experiencing North-South Syndrome or differential hypoxemia have several options: ECMO decannulation (if appropriate), conversion to V-V ECMO, or hybrid configurations like V-A-V ECMO.
Patients on V-A ECMO can also end up with acute lung injury and alveolar hemorrhage as a result of worsening pulmonary congestion from poor cardiac output, also known as ECMO Lung. Patients may require inotropes, an intraaortic balloon pump, or an Impella to improve cardiac contractility.
ECMO in the Literature
Studying ECMO has been challenging with varying levels of success. Studies have not been able to show unequivocal data for the benefit of V-V or V-A ECMO. Developing randomized control trials are difficult due to the diverse etiologies of cardiac arrest and respiratory failure. In addition, the wide practice variation between facilities adds to possible confounding variables. Crossover is also a major issue in ECMO trials, as it is understandably difficult for clinicians to allow severely ill patients to die from refractory shock or hypoxemia when randomized to non-ECMO care. In the early days, numerous case reports were published demonstrating anecdotal success which later evolved into large RCTs. Here we list landmark trials and their impact on ECMO development.
- “Extracorporeal membrane oxygenation in severe acute respiratory failure: A randomized prospective study”17 was published in 1979. This study compared 42 patients receiving partial V-A ECMO with mechanical ventilation with 48 patients receiving conventional mechanical ventilation for severe acute respiratory failure. Results did not show a difference, showing 9.5% versus 8.3% survived in the ECMO vs. conventional group. During this time, ECMO was being expanded and this paper is credited with deterring research efforts and enthusiasm for decades. However, this paper did act as one of the initial RCTs involving ECMO. It also highlighted potential areas of improvement of V-V over partial V-A with mechanical ventilation for respiratory only failure.
- In 2009, “Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomized trial” was published.18 This was a large, multicenter RCT that randomized patients with severe ARDS to transfer to a tertiary center for consideration of V-V ECMO or staying at a local hospital. The primary outcome was death or severe disability at 6 months. In brief, 180 patients were enrolled to randomly receive transfer with consideration for treatment with ECMO versus conventional management at a local center. The authors found that 63% of patients in the ECMO group survived to 6 months compared to 47% for the conventional management group (relative risk 0.69, 95% CI 0.05-0.97, p=0.03). This was one of the first studies to show a benefit of V-V ECMO for respiratory failure. The major criticism of this trial is now known as the “CESAR effect”: it was suspected that transfer to an experienced, tertiary center led to benefit independent of ECMO. This was reinforced by a subanalysis of the 76% of transferred patients who received ECMO, which demonstrated no difference from conventional care. Regardless, the CESAR trial is considered one of the most important and frequently cited ECMO trials to date.19
- An additional study in 2009 from Australia and New Zealand reported further positive results for V-V ECMO. “Extracorporeal Membrane Oxygenation for 2009 Influenza A (H1N1) Acute Respiratory Distress Syndrome” was an observational study of patients with influenza associated ARDS patients.20 A group of 68 patients were treated with ECMO and 71% had survived to ICU discharge with an approximate 21% mortality rate. Further meta-analysis of studies around the H1N1 virus outbreak demonstrated that ECMO was feasible to successfully combat ARDS caused by Influenza A (H1N1). Together, CESAR and the ANZ-ECMO series are credited with the success and further spread of V-V ECMO.19
- In 2018, the “Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome” (EOLIA) trial was published.21 This trial was a multicenter randomized control trial comparing patients with severe ARDS using immediate V-V ECMO versus conventional treatment. The primary endpoint was mortality at 60 days. The results showed that 35% of patients in the ECMO group and 46% of patients in the control group had died at 60 days (RR 0.76, 95% CI 0.55 to 1.93, P= 0.09). Although the study showed a decrease in mortality between the two groups, the result was not statistically significant. Further analysis showed that ECMO had a lower relative risk of treatment failure when compared to the control group. Treatment team failure was defined as death by day 60 or crossover to the ECMO group. In fact, 28% of the control patients crossed over for rescue ECMO as their clinical picture worsened. Of these, about 43% of patients in the crossover to ECMO group survived. Although the study did not show statistical significance, it does demonstrate that ECMO is not associated with an increase of death and may be more beneficial when used early for patients with severe ARDS.
- The “Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): a phase 2, single center, open-label, randomized controlled trial”22 studied ECMO in out-of-hospital cardiac arrest. This was the first large RCT for eCPR. This study had two groups of patients in cardiac arrest with a shockable rhythm, an ECMO group that went immediately to the cath lab to get placed on V-A ECMO at arrival versus a group that received standard ACLS. The primary outcome was survival to hospital discharge. The trial was stopped by the Data and Safety Monitoring Board after 30 patients. Results were encouraging with 43% vs 7% surviving to hospital discharge in the ECMO vs standard therapy group respectively (risk difference 36%, 3.7-59.2%; 0.986 probability of ECMO superiority) for the ECMO vs standard group respectively. Although it was a small single-center trial, this study generated significant momentum for eCPR.
- A 2023 study, “Early Extracorporeal CPR for Refractory Out-of-Hospital Cardiac Arrest (INCEPTION)”, again compared eCPR vs. conventional CPR.23 This was a larger, multicenter randomized control trial when compared to the ARREST or similar Prague OHCA study. INCEPTION once again compared eCPR for refractory cardiac arrest with an initial shockable rhythm with the primary outcome being favorable neurologic outcome at 30 days. The results showed that 20% of the 70 patients who received eCPR had a favorable neurologic outcome as compared to 16% of the patients who received traditional CPR (OR 1.4, CI 0.5 to 3.5; p=0.52). Benefit of eCPR was not found to be statistically significant in this study. Criticisms of this study include the longer times from arrest to cannulation (mean 74 minutes), early withdrawal of life-sustaining therapies, and the low case volume at each participating center.
As one can see, the evidence for ECMO has been conflicting from the beginning. Multiple challenges arise when conducting an RCT involving ECMO including finding consistent patient populations, indications, defining outcomes, and having similar execution strategies. There may never be consistent studies that definitively show ECMO benefit over conventional treatments. However, it is certain that ECMO has benefit in selected patients with cardiac and respiratory failure.
ECMO: Looking Toward the Future
ECMO has come a long way. Today, a subset of patients may walk, eat, and talk while on life support. Some emergency departments have an ECMO team available that can emergently cannulate a patient in cardiac arrest and put onto eCPR. A few regions even offer mobile ECMO units which allow for cannulation in the field. Select patients may live on ECMO for over a year as they receive treatment or as a bridge until possible solid organ transplant.
ECMO has great potential and is already a major part of many healthcare systems. Improvements in technology are set to radically change ECMO as we know today. ECMO technology is currently being developed into implantable “membrane lungs” in which one could wear and use the body's natural pressure to circulate blood through the oxygenator. Advanced coatings may reduce or eliminate the need for anticoagulation for patients.24 Improvements in oxygenators will continue to minimize surface area needed, making them even more mobile for patients. Servo-controlled ECMO pumps will reduce the need for constant monitoring and high-intensity staffing. The technology is even expanding into non-traditional areas such as ex-vivo perfusion in organ donation and artificial placentas. Going forward, further research is needed to identify which patients most benefit from ECMO cannulation and to address the cost of ECMO to the healthcare system.
Take-Home EM/Crit Care Points
- ECMO is an important tool in critical illness that can be used to improve oxygenation and/or provide circulatory support to our body’s vital organs through either V-V or V-A ECMO.
- If ECMO is available, it is important to identify patients early who may benefit from this therapy if they are rapidly decompensating or in cardiac arrest. It is important to know your local institutions' practices for ECMO.
- If a patient presents in cardiac arrest and may be a candidate for eCPR, consult your ECMO team early in the resuscitation as time to reperfusion is related to preserved neurologic function.
- ECMO is a team sport and requires significant interdisciplinary communication from critical care physicians, cardiothoracic surgeons, emergency medicine physicians, bedside nurses, respiratory therapists, perfusionists, transport teams, ECMO program leadership and more.
References
- Bauer TM, Tchantchaleishvili V. The Person Behind the Inventor of the Heart-Lung Machine: John H. Gibbon Jr, MD (1903-1973). Artif Organs. 2018;42(8):765-775. doi:10.1111/aor.13280
- ECLS Registry Report International Summary, April 2023, ELSO
- Aird WC. Discovery of the cardiovascular system: from Galen to William Harvey. J Thromb Haemost. 2011 Jul;9 Suppl 1:118-29. doi: 10.1111/j.1538-7836.2011.04312.x. PMID: 21781247.
- Lim MW. The history of extracorporeal oxygenators. Anaesthesia. 2006;61(10):984-995. doi:10.1111/j.1365-2044.2006.04781.
- Featherstone PJ, Ball CM. The origins of cardiopulmonary bypass. Anaesth Intensive Care. 2018 Jul;46(4):351-353. doi: 10.1177/0310057X1804600401. PMID: 29966106.
- Yeager T, Roy S. Evolution of Gas Permeable Membranes for Extracorporeal Membrane Oxygenation. Artif Organs. 2017;41(8):700-709. doi:10.1111/aor.12835
- Cohn LH. Fifty years of open-heart surgery. Circulation. 2003;107(17):2168-2170. doi:10.1161/01.CIR.0000071746.50876.E2
- Hsu J, Wang CH, Huang SC, Yu HY, Chi NH, Wu IH, Chan CY, Chang CI, Wang SS, Chen YS. Clinical Applications of Extracorporeal Membranous Oxygenation: A Mini-Review. Acta Cardiol Sin. 2014 Nov;30(6):507-13. doi: 10.6515/acs20140821a. PMID: 27122828; PMCID: PMC4804844.
- Bartlett RH. Extracorporeal life support: history and new directions. ASAIO J. 2005;51(5):487-489. doi:10.1097/01.mat.0000179141.08834.cb
- J Thorac Dis. 2015 Jul; 7(7): E166–E176. doi: 10.3978/j.issn.2072-1439.2015.07.17 PMCID: PMC4522501PMID: 26380745 Extra Corporeal Membrane Oxygenation (ECMO) review of a lifesaving technology
- Wrisinger WC, Thompson SL. Basics of Extracorporeal Membrane Oxygenation. Surg Clin North Am. 2022 Feb;102(1):23-35. doi: 10.1016/j.suc.2021.09.001. PMID: 34800387; PMCID: PMC8598290.
- Keebler ME, Haddad EV, Choi CW, et al. Venoarterial Extracorporeal Membrane Oxygenation in Cardiogenic Shock. JACC Heart Fail. 2018;6(6):503-516. doi:10.1016/j.jchf.2017.11.017
- Yeung Ng, P, Christin Carrol, et al; ECMO 101: Introductory Modules; https://www.elso.org/ecmo-education/ecmo-101-modules.aspx
- Zangrillo A, Landoni G, Biondi-Zoccai G, et al. A meta-analysis of complications and mortality of extracorporeal membrane oxygenation. Crit Care Resusc. 2013;15(3):172-178.
- Rabah H, Rabah A. Extracorporeal Membrane Oxygenation (ECMO): What We Need to Know. Cureus. 2022 Jul 11;14(7):e26735. doi: 10.7759/cureus.26735. PMID: 35967165; PMCID: PMC9363689.
- Makdisi G, Wang IW. Extra Corporeal Membrane Oxygenation (ECMO) review of a lifesaving technology. J Thorac Dis. 2015 Jul;7(7):E166-76. doi: 10.3978/j.issn.2072-1439.2015.07.17. PMID: 26380745; PMCID: PMC4522501.
- Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA. 1979;242(20):2193-2196. doi:10.1001/jama.242.20.2193
- Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial [published correction appears in Lancet. 2009 Oct 17;374(9698):1330]. Lancet. 2009;374(9698):1351-1363. doi:10.1016/S0140-6736(09)61069-2
- Sidebotham D. Extracorporeal membrane oxygenation--understanding the evidence: CESAR and beyond. J Extra Corpor Technol. 2011;43(1):P23-P26.
- Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators, Davies A, Jones D, et al. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA. 2009;302(17):1888-1895. doi:10.1001/jama.2009.1535
- Combes A, Hajage D, Capellier G, et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N Engl J Med. 2018;378(21):1965-1975. doi:10.1056/NEJMoa1800385
- Yannopoulos D, Bartos J, Raveendran G, et al. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): a phase 2, single centre, open-label, randomised controlled trial. Lancet. 2020;396(10265):1807-1816. doi:10.1016/S0140-6736(20)32338-2
- Suverein MM, Delnoij TSR, Lorusso R, et al. Early Extracorporeal CPR for Refractory Out-of-Hospital Cardiac Arrest. N Engl J Med. 2023;388(4):299-309. doi:10.1056/NEJMoa2204511
- Bartlett, R. ECMO: The Next Ten Years; The Egyptian Journal of Critical Care Med. 2016; vol 4,7-10. doi.org/10.1016/j.ejccm.2016.01.003.



