A 90-year-old male with a past medical history of severe aortic stenosis, heart failure with reduced ejection fraction, and chronic kidney disease presents with shortness of breath. His vital signs are as follows: HR 112, BP 85/60, RR 28, SpO2 85% on room air. Bedside ultrasound reveals diffuse B lines consistent with pulmonary edema, and he is placed on non-invasive positive pressure ventilation.
What is the best way to manage this patient, particularly if he continues to decompensate?
Critical aortic stenosis (AS) is the single most problematic valvular disease we encounter in the emergency department. Patients with critical AS have a fixed cardiac output and cannot meaningfully increase cardiac output to meet the physiologic demands of critical illness. Avoiding systemic hypotension, maintaining sinus rhythm, and avoiding excessive tachycardia are therefore the cornerstones of resuscitation.
Background
AS is the third most common cardiovascular disease in the developed world, eclipsed only by systemic hypertension and coronary artery disease. The prevalence in the general population is 0.4%, but increases to 9.8% in octogenarians, with an overall prevalence of 2.8% in adults older than 75 years of age.1,2 Valve replacement, either surgical or catheter directed (ie, transcatheter aortic valve replacement, or TAVR), is the mainstay of treatment for advanced disease.
In a normal adult, the aortic valve area measures 2.6 to 3.5 cm2. AS becomes hemodynamically significant when aortic valve area approaches <1 cm2. As the valve becomes tighter, the pressure gradient across the valve increases. A pressure gradient >50 mmHg indicates severe disease.3-5 However, it is important to note that a substantial proportion of patients with severe and critical AS have a low gradient, which most frequently results from the decreased stroke volume associated with advanced disease.
Hemodynamically significant AS must be on the differential in the undifferentiated patient presenting with acute pulmonary edema, syncope, or cardiogenic shock, particularly if they are elderly. In addition to the identification of a systolic ejection murmur, bedside echocardiography can help screen patients. In fact, qualitative assessment of the aortic valve from the parasternal long and short axis views has been shown to be 75% sensitive and 93% specific for the diagnosis of severe AS among trained emergency medicine providers.6
Physiology Primer
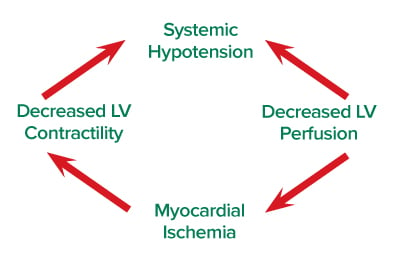
The stenotic aortic valve results in a buildup of pressure inside the left ventricle and a comparably lower pressure in the aortic root, resulting in low coronary perfusion pressure. This leaves the left ventricle uniquely susceptible to ischemia, which reduces cardiac output and promotes further ischemia.7 Systemic hypotension reduces coronary perfusion pressure, and excessive tachycardia increases myocardial oxygen demand; both contribute to a self-perpetuating cycle of ischemia and cardiogenic shock (see Figure).
The left ventricle hypertrophies in critical AS in response to chronically increased afterload. A stiff, hypertrophied left ventricle requires high filling pressures, and the “atrial kick” of sinus rhythm to fill in diastole. Hypovolemia and supraventricular tachyarrhythmias (eg, atrial fibrillation) dramatically reduce left ventricular preload and are tolerated poorly in this patient population.8 Excessive tachycardia not only promotes ischemia, but also reduces time spent in diastole for left ventricular filling.
Vasopressor Management
Phenylephrine is the vasopressor of choice in treating the hypotensive patient with AS. Using an agent that solely increases afterload is initially counterintuitive. It is important to recognize that the massive afterload of aortic stenosis is at the level of the aortic valve, with little contribution from the systemic vasculature. As a pure alpha-1 agonist, phenylephrine increases diastolic blood pressure and thus improves coronary perfusion. Phenylephrine also may result in a reflex bradycardia — a favorable pharmacodynamic property for its use in aortic stenosis.9 Norepinephrine is, similarly, a reasonable choice. Avoid epinephrine as a first line agent given its strong beta-1 agonism and propensity to promote tachycardia and increase myocardial oxygen demand.
Fluid Management
The tight aortic valve increases left-sided pressures and can lead to pulmonary congestion. However, patients with AS also have diastolic dysfunction and depend on preload to fill the left ventricle and maintain cardiac output. Decisions on fluid administration are challenging in this patient population and should be made in the context of the clinical scenario.
Consider the hemodynamically unstable patient with critical AS who you
are preparing to intubate. Any concern regarding pulmonary congestion is superseded by the need to optimize preload prior to induction. Assuming
the patient is not on the tail end of the Frank-Starling curve, temporarily infusing crystalloids to optimize preload and stave off peri-intubation hypotension is a good idea.
Now, consider a stable patient with critical AS who is having a slow GI bleed. So long as there are no hemodynamic perturbations to necessitate rapid replacement of blood products, the clinical scenario calls for a cautious fluid administration strategy because the patient is prone to developing pulmonary edema. Administer small volumes, and frequently reassess for signs of congestion (eg, B lines on lung ultrasound).
Intubation
Patients with critical AS depend on adequate left ventricular preload to maintain cardiac output. Both induction agents and positive pressure ventilation acutely drop left ventricular preload, and place patients with critical AS at risk for peri-intubation hemodynamic collapse. Hemodynamic optimization prior to induction, adequate monitoring, and selection of an induction agent with a favorable hemodynamic profile are the mainstays of safe intubation.
As you prepare to intubate, have push-dose phenylephrine at the bedside (or infusing). Prior to induction, the patient should be preload optimized. If there is any uncertainty regarding volume status, err on the side of fluid administration. Even short periods of systemic hypotension can be devastating and are easily missed by a periodically cycling non-invasive blood pressure cuff. If time allows (eg, the urgent-not-emergent intubation), consider placing an arterial line prior to induction to decrease response time to systemic hypotension.
Patients with critical AS depend on the atrial kick of sinus rhythm for diastolic filling. If you are preparing to intubate a hemodynamically tenuous patient in new atrial fibrillation (or any SVT for that matter), consider cardioversion prior to induction.
Etomidate is the RSI induction agent of choice in patients with AS, because it is both hemodynamically stable and comes with a generally favorable side effect profile. Propofol is a profound vasodilator and can acutely drop preload and promote hemodynamic collapse. Ketamine promotes tachycardia — an unfavorable property in patients with AS.
Case Conclusion
The patient was placed on a phenylephrine infusion, and preload was optimized prior to intubation. After intubation he was admitted to the CCU, where he was cautiously diuresed throughout the next week. He responded well to medical management and was extubated without event. Ultimately, he was transferred to another institution for TAVR and had a full recovery.
Definitive management of AS is generally surgical or endovascular, and is informed by formal echocardiography. However, ED providers will no doubt encounter patients with previously unrecognized AS. Physical exam and bedside echocardiography can suggest the diagnosis of AS when little background clinical data is available. Patients with hemodynamically significant AS are challenging to manage in the resuscitation phase. Avoid excessive tachycardia, maintain sinus rhythm, optimize preload, and treat hypotension aggressively with phenylephrine.
References
- Eveborn GW, Schirmer H, Heggelund G, Lunde P, Rasmussen K. The evolving epidemiology of valvular aortic stenosis. the tromso study. Heart. 2013;99(6):396-400.
- Lindman BR, Clavel M, Mathieu P, et al. Calcific aortic stenosis. Nature Reviews Disease Primers. 2016;2:nrdp20166.
- Frank S, Johnson A, Ross J,Jr. Natural history of valvular aortic stenosis. Br Heart J. 1973;35(1):41-46.
- Kennedy KD, Nishimura RA, Holmes DR, Bailey KR. Natural history of moderate aortic stenosis. J Am Coll Cardiol. 1991;17(2):313-319.
- Turina J, Hess O, Sepulcri F, Krayenbuehl H. Spontaneous course of aortic valve disease. Eur Heart J. 1987;8(5):471-483.
- Alzahrani H, Woo MY, Johnson C, Pageau P, Millington S, Thiruganasambandamoorthy V. Can severe aortic stenosis be identified by emergency physicians when interpreting a simplified two-view echocardiogram obtained by trained echocardiographers? Critical ultrasound journal. 2015;7(1):5.
- Hensley FA, Martin DE, Gravlee GP. A practical approach to cardiac anesthesia. Lippincott Williams & Wilkins; 2012.
- Horak J, Weiss S. Emergent management of the airway: New pharmacology and the control of comorbidities in cardiac disease, ischemia, and valvular heart disease. Crit Care Clin. 2000;16(3):411-427.
- Goertz AW, Lindner KH, Schutz W, Schirmer U, Beyer M, Georgieff M. Influence of phenylephrine bolus administration on left ventricular filling dynamics in patients with coronary artery disease and patients with valvular aortic stenosis. Anesthesiology. 1994;81(1):49-58.