Individuals commonly wear commercially available fitness trackers to monitor physical activity, record exercise metrics, and stay connected to their primary devices. The Apple Watch, for example, has advanced capabilities that include heart rate monitoring, blood oxygen level measurement, body temperature tracking, and ECG recording.
As these devices become more accessible, widespread, and sophisticated, they increasingly play a vital role in emergency diagnosis and medical management.
To record an ECG using the device, the wearer must manually initiate the recording. Data is then recorded and transmitted to the wearer’s smart device, allowing the report to be shared, saved, and further analyzed. The Apple Watch is primarily designed to identify atrial fibrillation (Afib).1 However, research has demonstrated that the Apple Watch’s ECG feature has similar monitoring capabilities to a 12-lead ECG when interpreted by a medical professional.1-3
Adalimumab is a monoclonal antibody used to treat various autoimmune and inflammatory diseases by blocking inflammatory cytokines like TNF-alpha. Several cases have highlighted the potential risk of cardiotoxicity associated with TNF-alpha inhibitors like adalimumab.4-6 In our case, ischemic and infectious etiologies were ruled out, leading the medical team to consider adalimumab as a possible cause.4,5
Case Report
A 36-year-old patient with Crohn’s disease on adalimumab since 2019 presented to the ED after an episode of palpitations during exercise. He was wearing a smartwatch with ECG capabilities and recorded the episode (Image 1). The patient, who happened to be an emergency physician, then reviewed the tracing, which was concerning for NSVT. His only other symptom at the time was shortness of breath, but he said this was baseline for his exercise routine. He did not report presyncope or loss of consciousness. He had no reported history of tobacco, alcohol, or substance abuse. He said he consumed 1 cup of coffee per day. He did report a family history of coronary artery disease.
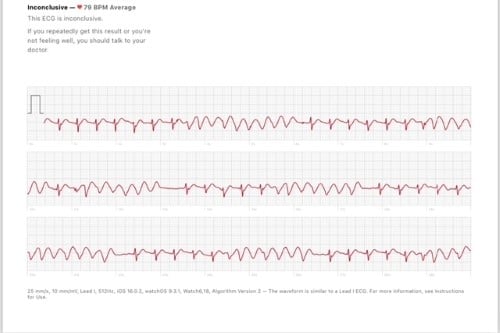
Image 1
On initial examination, he was well-appearing and in no acute distress. His blood pressure was 126/89, heart rate 80, respiratory rate 14, and SpO2 99% on room air.
Initial ECG showed sinus rhythm with an early repolarization pattern. Labs revealed BUN 21, creatinine 1.1, sodium 141, potassium 4.2, chloride 105, magnesium 1.6, phosphorus 2.1, creatinine kinase 109, and high-sensitivity troponin 4. Magnesium supplementation was administered in the ED.
Cardiology evaluated the patient in the ED and recommended a stat echocardiogram and coronary CTA. The echocardiogram reported normal LVEF, normal biventricular function, and no gross valvular pathology. Coronary CTA revealed a calcium score of 0 with normal coronary arteries.
During his ED stay, no other events were noted on telemetry monitoring, and he remained asymptomatic. He was then discharged on metoprolol 25mg twice per day, a 14-day Holter monitor device, and a follow-up for cardiac MRI.
A cardiac MRI revealed normal right ventricle (RV) size, mildly reduced RV systolic function, and global hypokinesis. The RV ejection fraction was 33% (normal range for men, 42%-72%).
During a routine follow-up with his gastroenterologist, it was discussed that there were reports of left ventricular heart failure with adalimumab use. The patient was changed to a different biologic and remained asymptomatic.
Discussion
Several reports have demonstrated the potential of smartwatches in detecting symptomatic arrhythmias.1-3 These devices can provide valuable information, but they have limitations. For example, detecting ventricular tachycardia and other arrhythmias might not always be accurate or comprehensive, and some arrhythmias may go undetected.1 Additionally, activating the smartwatch’s ECG function requires patient awareness of symptoms and manual operation, which can be challenging during asymptomatic or severe arrhythmia episodes.3
Our case underscores the necessity for more robust clinical studies and consensus statements on the use of wearable technology in arrhythmia detection and management. Guidelines should address limitations and offer recommendations on how health-care professionals should interpret and act on data provided by smartwatches.
Additionally, this case report contributes to the growing body of evidence suggesting that TNF-alpha inhibitors may carry a risk of cardiotoxicity.4,5 In this case, the cardiac arrhythmia was attributed to adalimumab-induced heart failure, an unusual complication of the drug.
Further research into the cardiotoxicity profile of TNF-alpha inhibitors like adalimumab is necessary so that we can better understand the mechanisms involved and potential risk factors. Research could guide the development of safer therapeutic approaches and help clinicians make informed decisions regarding the use of these drugs.
We urge physicians to carefully monitor patients on TNF-alpha inhibitors for signs of cardiotoxicity and encourage health-care professionals to consider the possibility of drug-induced cardiotoxicity when evaluating patients with new-onset heart failure.
This case demonstrates a unique intersection between modern technology, contemporary pharmacotherapy, and emergency medicine. Early detection of symptoms like shortness of breath and palpitations can lead to prompt intervention and potentially prevent severe outcomes.
The use of health data from a smart device has shown significant diagnostic utility and will continue to do so as technology advances.1-3 Emergency physicians should be aware of these potential monitoring tools; formal guidelines are necessary to integrate this technology into daily practice.
References
- Perino AC, Gummidipundi SE, Lee J, et al. Prevalence of Arrhythmias Other Than Atrial Fibrillation in Those With an Irregular Pulse Detected on a Smartwatch. Circ Arrhythm Electrophysiol. 2021;14(10):e010063. doi:10.1161/CIRCEP.121.010063.
- Alnasser S, Alkalthem D, Alenazi S, Alsowinea M, Alanazi N, Al Fagih A. The Reliability of the Apple Watch's Electrocardiogram. Cureus. 2023 Dec 1;15(12):e49786. doi: 10.7759/cureus.49786. PMID: 38161560; PMCID: PMC10757793.
- Burke J, Haigney MCP, Borne R, Krantz MJ. Smartwatch detection of ventricular tachycardia: Case series. HeartRhythm Case Rep. 2020;6(10):800-804. doi:10.1016/j.hrcr.2020.08.003. PMID: 33101960; PMCID: PMC7573479.
- Toufaily A. Severe cardiomyopathy induced by Adalimumab administration for Crohn's disease. J Cardio Case Rep. 2020;3:129. doi:10.15761/JCCR.1000129.
- Mansitó López C, Torres Laboy P, Ortiz Bou M, Quintero Noriega A, Cintron Rivera V. Fatal New-Onset Congestive Heart Failure Related to Adalimumab Use in a Patient with Relapsing Hidradenitis Suppurativa: A Case Report. Am J Case Rep. 2021 Feb 10;22:e929148. doi: 10.12659/AJCR.929148. PMID: 33563886; PMCID: PMC7883937.
- Morgenweck E, et al. Heart failure associated with ustekinumab therapy for treatment of Crohn's disease. Am J Gastroenterol. 2021;116(Suppl.):S1043. doi:10.14309/01.ajg.0000783412.67823.2f.



