As the coronavirus outbreak continues to develop, the CDC has released interim guidelines that all emergency physicians should note.
The World Health Organization (WHO) declared the novel coronavirus (2019-nCoV) to be a public health emergency of international concern on Jan. 30, 2020.6 The 2019-nCoV refers to the tentative name given to the new strain of coronavirus that was first encountered in Wuhan, China.6 In December 2019, an outbreak of viral pneumonia in Wuhan prompted the discovery of a novel coronavirus, which was traced back to a seafood market in the region.3,1,10 Since then, there have been multiple confirmed 2019-nCoV cases in Asia, Australia, Europe, and North America.6,3,1
Coronavirus is an enveloped, positive sense, single stranded RNA virus that is a member of the coronaviridae family.1,10 Other coronaviruses include the Middle East Respiratory Syndrome CoV (MERS CoV) and the Severe Acute Respiratory Syndrome CoV (SARS CoV).6,1,3,4 The SARS CoV outbreak started in China in 2002, causing more than 8000 infections and claiming the lives of 800 people before it was contained in 2003.6,3,1 Its primary reservoir was bats.1 The 2019-nCoV shows genetic similarity to the SARS virus, suggesting its origin may also be from bats.7 The MERS CoV, on the other hand, was first reported in Saudi Arabia in 2012 and its primary reservoir was camels.1 Both MERS and SARS had their origins in animals and eventually progressed to human-to-human transmission.1,10
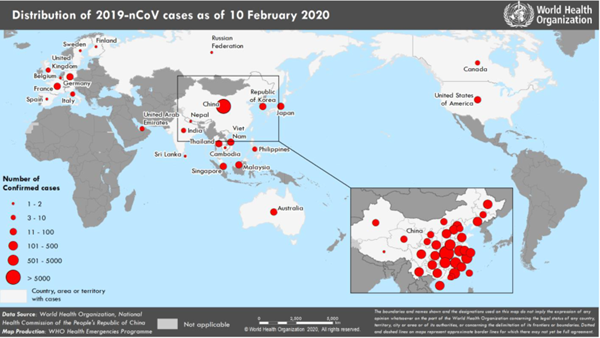
On Jan. 4, 2020, China placed a quarantine on Wuhan and the surrounding areas to prevent the spread of disease.8 As of Feb. 10, there were 40,554 confirmed 2019-nCoV cases globally with 910 reported deaths, only 1 of which was outside of China (Image 1).5
The United States reported its first confirmed 2019-nCoV case on Jan. 21, 2020.6 As of Feb. 14, there were 15 confirmed cases in the United States located in Arizona, California, Illinois, Massachusetts, Texas, Wisconsin, and Washington.6
Signs and Symptoms
The CDC reports that patients may become symptomatic anywhere from 2-14 days after 2019-nCoV exposure, which was the case with the MERS virus.6 The typical presentation of 2019-nCoV in the ED includes fever (83-98% of cases), cough (76-82%), myalgias or fatigue (11-44%), and shortness of breath (>20%).1,9 Other symptoms that have been reported include sore throat, productive cough, headache, hemoptysis, and diarrhea.6 The severity of presentation has ranged from asymptomatic to severe illness and even death. To date, no specific risk factors have been identified, but more severe presentations have been reported in patients who have existing comorbidities such as diabetes mellitus, hypertension, or cardiovascular disease.6 Complications include pneumonia as well as acute respiratory distress syndrome (ARDS) (17-29% of hospitalized patients), acute cardiac injury (12%), and acute kidney injury (4-7%).6 Laboratory abnormalities among patients hospitalized with pneumonia include nonspecific markers such as leukopenia (9-25%), leukocytosis (24-30%), lymphopenia (63%), and transaminitis (37%).6 Imaging of the chest often shows bilateral findings and includes consolidation or ground glass opacities.6
Interim CDC Guidelines
The CDC posted interim guidelines to evaluate patients with suspected 2019-nCoV infections. The guidelines state that the 2019-nCoV should be considered if patients present with any of the following criteria (Figure 2):
- Fever or signs/symptoms of lower respiratory illness AND had close contact with a confirmed 2019-nCoV within 14 days of symptoms onset
- Fever and signs/symptoms of lower respiratory illness AND had history of travel from Hubei, China within 14 days of symptoms onset
- Fever and signs/symptoms of lower respiratory illness requiring hospitalization AND had history of travel from mainland China within 14 days of symptoms onset.6
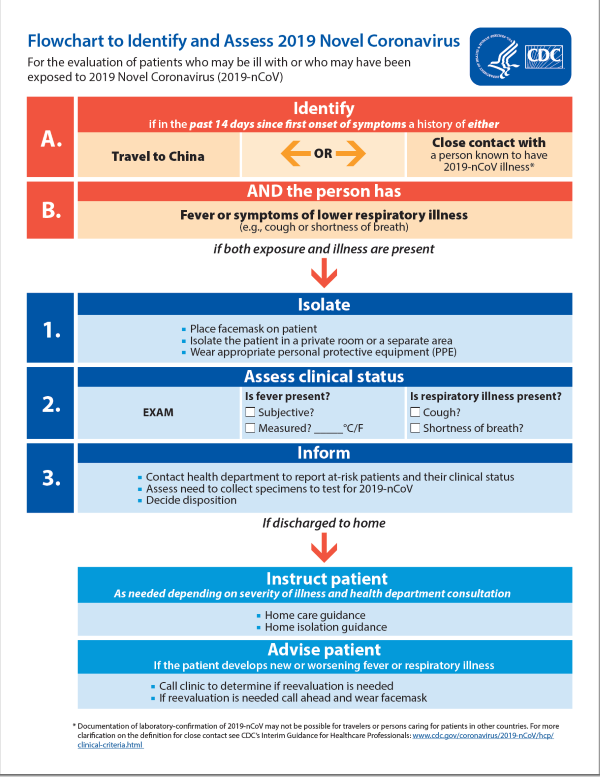
The CDC further characterizes the fever as subjective or confirmed. It describes “close contact” with an infected individual as: either being within 6 feet (2 meters) of a 2019-nCoV infected individual without wearing personal protective equipment or having direct contact with respiratory secretions (such as cough droplets) of an infected individual without having the proper personal protective equipment.6 Examples of lower respiratory illness symptoms include cough and shortness of breath.6
Patients meeting these criteria should be placed in isolation. The CDC recommends these patients be placed in an airborne infection isolation room in addition to a facemask. Moreover, any person coming in contact with the patient should wear personal protective equipment to include eye protection.6,2
If a patient is under consideration for 2019-nCoV, health care providers should immediately contact the infection control department at their facility as well as their local or state health department without waiting for results of any testing.6 The state health department will in turn get into contact with the CDC’s Emergency Operations Center (EOC) in order to complete a 2019-nCoV Person Under Investigation form.6 The 2019-nCoV isolation and diagnostic testing (using RT-PCR) can only be done at the CDC.6 The EOC determines the need for testing as well as assistance from the local health department in collecting, storing, and transporting specimens to the CDC.6 2019-nCoV specimens should be collected from upper (nasopharyngeal or oropharyngeal) and lower respiratory (sputum) airways for higher rates of detecting the virus.6 Local virus testing and isolation is not recommended because of biosafety reasons.6
Emergency physicians should evaluate and test for alternative causes while awaiting EOC recommendations. A respiratory viral panel, including influenza, can help rule out other viral etiologies including coronavirus HKU1, another member of the coronaviridae family. Testing for other causes should not delay the investigation for 2019-nCoV.6
Treatment
The treatment for 2019-nCoV is supportive and aimed at symptom management and prevention of complications.6,2 Corticosteroids should be avoided due to concern of prolonging the viral replication as previously seen with the MERS outbreak.6 Antiviral medications such as neuraminidase inhibitors (oseltamivir), ribonuclease analogs (ribavirin for example), or interferons are not likely to be effective in treating 2019-nCoV.10,4 Future treatments that may be effective for 2019-nCoV are monoclonal antibodies which have been successful in treating SARS CoV and MERS CoV.4,10 Other drugs that may be effective in treatment include remdesivir, lopinavir/ritonavir, lopinavir/ritonavir combined with interferon-β, convalescent plasma - but research is still needed to confirm outcomes.4
Disposition
Decisions on patient disposition are made on a case by case basis with the careful discussion between health care providers and public health representatives.6 Considerations for discontinuing transmission precautions depend on the severity of patients’ symptoms and results of the 2019-nCoV testing.6 The current CDC guidelines state that transmission precautions can only be discontinued when meeting all these criteria:
- Resolution of fever without using antipyretic medications
- Improvement of signs and symptoms
- Negative rT PCR 2019-nCoV testing from at least 2 sequential respiratory (nasopharyngeal and throat swabs) samples that were taken at least 24 hours apart.6
If the decision is made to discharge the patient home, careful instructions must be given to patients regarding when to come back to the hospital. Patients should be instructed to return to the hospital if their symptoms worsen and to do so while wearing a facemask. Worsening of symptoms has been reported more often in the second week after symptoms onset, with more complications in the elderly, immunocompromised, and those with comorbidities.6
Research to isolate and characterize the 2019-nCoV is ongoing. The main goals of emergency department management are viral containment, symptom management, and exposure prevention. Health care providers are most at risk in the emergency department setting due to direct contact with infected individuals. Close adherence to the CDC guidelines for infection control of the 2019-nCov is imperative and ensures decreased transmission and isolation of infected individuals.
References
- Chan JF-W, Kok K-H, Zhu Z, et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9(1):221-236.
- Covid-19 (Coronavirus) Clinical Alert. Accessed Feb. 10, 2020.
- Hui DS, Azhar EI, Madani TA, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health — The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264-266.
- Li H, Wang YM, Xu JY, Cao B. Potential antiviral therapeutics for 2019 Novel Coronavirus. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(0). Published online ahead of print. Accessed Feb. 10, 2020.
- World Health Organization. Novel Coronavirus (2019-nCoV) situation reports. Accessed Feb. 10, 2020.
- Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19). Accessed Feb. 4, 2020.
- Paraskevis D, Kostaki E, Magiorkinis G, Panayiotakopoulos G, Sourvinos G, Tsiodras S. Full-genome evolutionary analysis of the novel corona virus (2019-nCoV) rejects the hypothesis of emergence as a result of a recent recombination event. Infect Genet Evol. 2020;79:104212.
- Tuite AR, Fisman DN. Reporting, Epidemic Growth, and Reproduction Numbers for the 2019 Novel Coronavirus (2019-nCoV) Epidemic. Ann Intern Med. 2020; Feb 5[Online ahead of print].
- Working Group of 2019 Novel Coronavirus, Peking Union Medical College Hospital. Diagnosis and clinical management of 2019 novel coronavirus infection: an operational recommendation of Peking Union Medical College Hospital (V2.0). Zhonghua Nei Ke Za Zhi. 2020;59(3):186-188.
- Yu F, Du L, Ojcius DM, Pan C, Jiang S. Measures for diagnosing and treating infections by a novel coronavirus responsible for a pneumonia outbreak originating in Wuhan, China. Microbes Infect. 2020. Published online ahead of print.