Article
Horby PW, Lim WS, Emberson J, et al. Effects of Dexamethasone in Hospitalized Patients with COVID-19 - Preliminary Report. Preprint via medRxiv: The Preprint Server for Health Sciences. 2020. medRxiv preprint doi: https://doi.org/10.1101/2020.06.22.20137273.
OBJECTIVE
To determine the effect of dexamethasone on 28-day mortality in hospitalized patients with COVID-19
BACKGROUND
While the COVID-19 pandemic has caused widespread global devastation, a treatment has not yet been identified that reduces mortality in patients with the disease. The first success in the search for a treatment came with the recent ACTT-1 Trial (https://www.emra.org/emresident/article/critcare-alert-remdesivir/), which found that the antiviral remdesivir reduces time to recovery for hospitalized COVID-19 patients but stopped just short of showing a mortality reduction.
There is a great deal of uncertainty about the role of corticosteroids in treating COVID-19. The disease process appears to start with an acute viral phase that, in some patients, transforms into a dysregulated inflammatory response in which the patient’s immune system is the major cause of harm. While antivirals such as remdesivir may help in the initial viral phase, corticosteroids may help diminish the later inflammatory response (https://www.emra.org/emresident/article/critcare-alert-dexa-ards/). They may, however, also blunt an appropriate immune response to the initial viral insult, increase viral shedding, or allow another opportunistic infection to take hold. Because of this uncertainty, practice patterns for corticosteroid administration have varied widely. They are recommended in some places while contraindicated in others.
It is important to note that this paper is a preprint, meaning it has not gone through peer review. The authors released these results prior to peer review because of their view that changes in practice should not wait for the normal peer review process during a rapidly evolving pandemic. This has become a more common practice in the past few months with various levels of credibility, but it should be noted that this study was conducted by a highly credible organization and was not funded by pharmaceutical companies with any financial interest in the drug. It should, however, be read with the consideration that it is not yet a journal article.
DESIGN
Open-label, randomized, controlled trial conducted at 176 NHS hospitals in the United Kingdom.
POPULATION
Hospitalized patients with clinically suspected or laboratory confirmed COVID-19. The age limit of >18 years old was removed mid-study in May 2020. Patients were excluded if their treating physician believed the treatment would put them at significant risk. Pregnant and breast-feeding patients were included.
INTERVENTION
Dexamethasone 6 mg daily for 10 days
CONTROL
Usual care, defined as: "Patient receives usual hospital care"
OUTCOMES
Primary
• 28-day all-cause mortality
Prespecified subgroup analyses were conducted based on:
• Age
• Sex
• Level of respiratory support
• Days since symptom onset
• Predicted 28-day mortality risk
Secondary
• Time to discharge
• Subsequent need for mechanical ventilation
• Death
KEY RESULTS
Overall, 2104 patients received dexamethasone and 4,321 patients received usual care. After randomization, mean age was 1.1 years higher in the dexamethasone group, and the final results were adjusted for this difference. This adjustment was not pre-specified, but unadjusted results were similar.
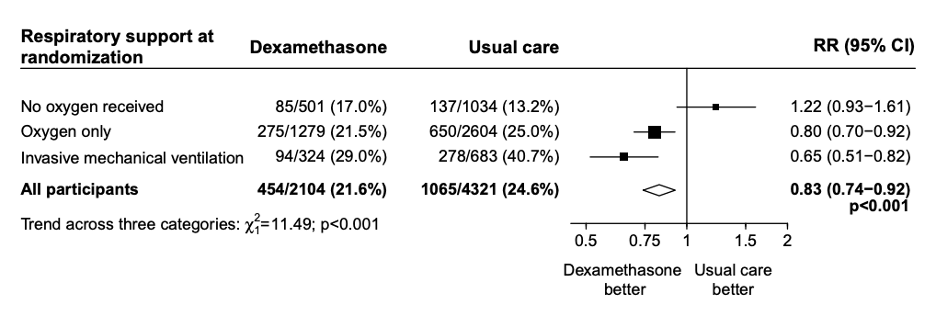
Patients in the dexamethasone group had significantly lower 28-day mortality (21.6% vs 24.6%, RR 0.83, 95% CI: 0.74-0.92). In the pre-specified subgroup analysis there was a significant trend (using chi-squared test) toward more benefit in patients needing the most respiratory support. Patients on invasive mechanical ventilation had a mortality reduction of one-third (RR 0.65, 95% CI: 0.51-0.82), patients receiving oxygen had a mortality reduction of one-fifth (RR 0.80, 95% CI: 0.70-0.92), while patients not requiring oxygen had no mortality benefit (RR 1.22, 95% CI: 0.93-1.61). Mortality was also reduced more in patients with longer duration of symptoms. The secondary outcomes of 28-day discharge and subsequent need for mechanical ventilation or subsequent death both showed benefit in the dexamethasone group.
FIGURE 1. The Kaplan Meier curves for each subgroup. Source: RECOVERY Collaborative Group
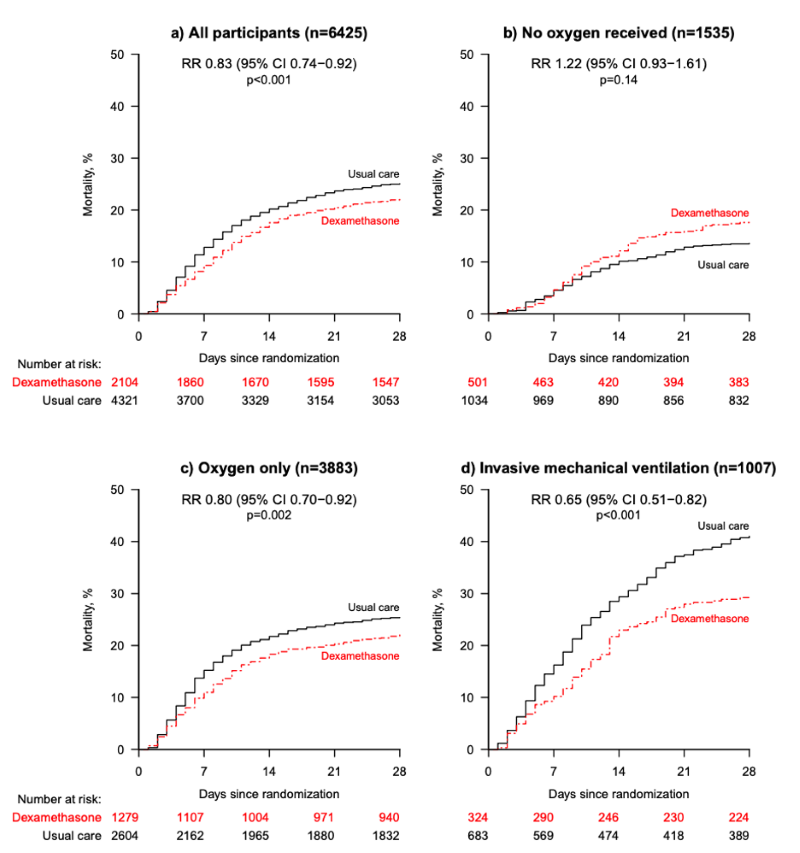
FIGURE 2. Mortality trend across subgroups. Source: RECOVERY Collaborative Group
STRENGTHS
• Well-designed, randomized controlled trial studying a question of genuine uncertainty
• Broad inclusion criteria: 15% of COVID patients hospitalized in the UK were included in the study
• Robust statistical support for conclusions drawn
LIMITATIONS
• Has not yet been peer-reviewed
• Not blinded
EM TAKE-AWAYS
This is the first study to identify a treatment for COVID-19 that reduces mortality. The mortality reduction is large, with a number needed to treat to prevent one death of 8 patients receiving mechanical ventilation and 25 patients requiring oxygen. There was, however, a nonsignificant signal of harm in patients who didn’t require oxygen support.
The mortality reduction is higher for patients requiring more ventilatory support and with longer duration of symptoms, which supports the theory that it is beneficial for the inflammatory, rather than viral, stage of the disease. The authors stress the need to treat with the "right dose, at the right time, in the right patient." Though dexamethasone is beneficial for patients in later stages of the disease, use in patients in the viral stage could cause harm by reducing resistance to viral replication. Dexamethasone should not be automatically added to a "COVID cocktail" used for every COVID patient, but should be given after careful consideration of benefits and harms in individual patients. Special care should especially be taken to avoid fueling a public narrative that this cheap, easily available drug is a "cure" for COVID-19 when patients with mild illness could be harmed by taking dexamethasone.
References
- Horby PW, Lim WS, Emberson J, et al. Effects of Dexamethasone in Hospitalized Patients with COVID-19 - Preliminary Report. Preprint via medRxiv: The Preprint Server for Health Sciences. 2020. medRxiv preprint doi: https://doi.org/10.1101/2020.06.22.20137273.




