ARTICLE: Gibbs KW, Semler MW, Driver BE, et al. Noninvasive Ventilation for Preoxygenation during Emergency Intubation. N Engl J Med. 2024;390(23):2165-2177.
OBJECTIVE: To evaluate the effect of preoxygenation with noninvasive ventilation compared to preoxygenation with an oxygen mask on hypoxemia during tracheal intubation among critically ill adults
BACKGROUND
Hypoxemia is a known complication during the peri-intubation period and increases the risk of cardiac arrest, death, and additional complications. The incidence of hypoxemia during intubations in the emergency department is surprisingly high, at around 10-20% despite preoxygenation efforts.1 Proxygenation is the administration of a high amount of supplemental oxygen before induction of anesthesia to increase oxygen partial pressure in the lung and delay the onset of hypoxemia during apnea. Preoxygenation techniques vary in practice, from nasal cannula to noninvasive ventilation (NIV). However, in most current clinical care, critically ill adults receive preoxygenation by an oxygen mask.1 Practice in oxygenation with standard face masks also varies as the rate and percentage of oxygen can vary based on the chosen flow rate (ie, 15 L/min vs flush rate (wide open valve)). Additional factors can make face masks less effective including poor fit and the lack of positive pressure.
NIV has distinct advantages in oxygenation over face masks. In addition to the high flow rate and increased fraction of inspired oxygen (FiO2), it provides positive pressure ventilation which can help prevent atelectasis, increase functional residual capacity, and help recruit poorly ventilated lung regions. These additional features could allow for increased oxygenation during the pre-intubation period.2 However, there are practical concerns such as additional time and resources needed to set up NIV. There is also a theoretical concern of an increased risk of aspiration with NIV5.
Two previous small, randomized trials have compared NIV with oxygen masks for preoxygenation in critically ill adults. One showed a lower risk of hypoxemia with NIV, while the other showed no statistical difference.2,3 Currently, guidelines state that preoxygenation with face masks or NIV are both acceptable, and clinical practice varies. The PREOXI trial is a large, pragmatic, randomized trial to determine the effect of preoxygenation with NIV compared to oxygen masks.
DESIGN
This was a pragmatic, multicenter, unblinded, randomized, parallel-group trial. Unlike an explanatory trial, which aims to confirm a hypothesis, pragmatic trials aim to demonstrate the feasibility of an intervention by providing information on its use in real-world clinical practice.4 Parallel-group design means participants are randomized to one or more study arms, and each study arm will receive a different intervention. In contrast to a cross-over study, here the participants stay in their assigned study arm during the whole study.
This study was conducted at 24 sites (7 emergency departments and 17 ICUs) at 15 medical centers in the United States. Patients were randomly assigned in a 1:1 ratio to receive preoxygenation with either NIV or an oxygen mask. Given the nature of the intervention, clinicians were aware of the trial-group assignments after randomization.
The diagram below summarizes the interventions during each phase of the intubation process. Patients underwent preoxygenation for at least 3 minutes before the induction of anesthesia (if feasible). For the NIV group, the settings were: FiO2 100%, EPAP of at least 5 cm H2O, IPAP of at least 10 cm H2O, and respiratory rate of at least 10 breaths per minute. NIV could be administered using a conventional mechanical ventilator or dedicated noninvasive ventilator. Oxygen was administered via non-rebreather mask or bag-mask device without ventilation for the oxygen-mask group For the oxygen mask group, the operator administered the highest flow rate of oxygen possible (>= 15 liters per minute).
The study arm assignment only applied to the initial method of preoxygenation before induction of anesthesia. In either group, the protocol allowed for the use of oxygen through the nasal cannula during any phase of the intubation procedure. The protocol recommended the use of the assigned method of preoxygenation between induction of anesthesia and initiation of laryngoscopy. As an alternative, the use of a bag-mask device with ventilation was also allowed for both trial groups in the period between induction of anesthesia and initiation of laryngoscopy.
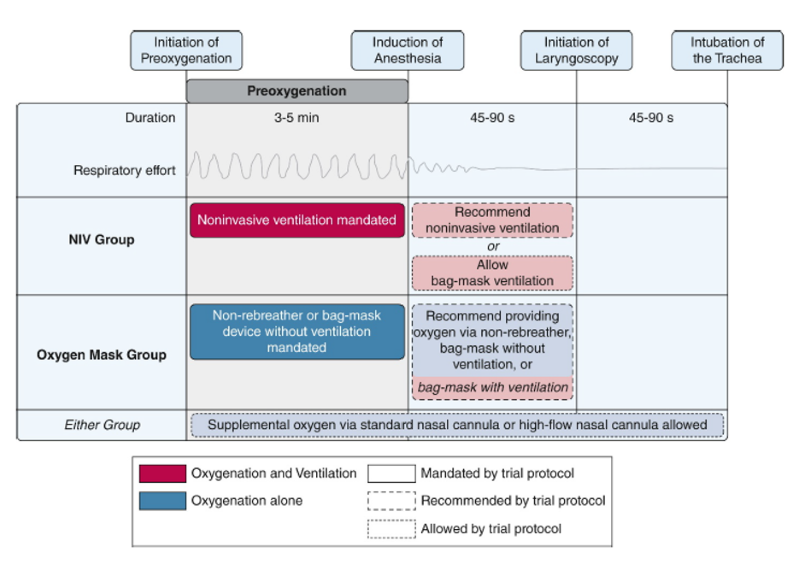 Figure 1. Trial interventions5
Figure 1. Trial interventions5
INCLUSION CRITERIA
- Patient is located in a participating unit: ICU or ED
- Patients undergoing a tracheal intubation that involved using a laryngoscope and sedation
- Planned operator is a clinician expected to routinely perform tracheal intubation in the participating unit
EXCLUSION CRITERIA
- Patient was already receiving positive pressure ventilation by a mechanical ventilator, bag-mask device, or laryngeal mask airway
- Less than 18 years old
- Pregnant
- Prisoner
- Immediate need for tracheal intubation that precluded randomization
- Patient is apneic or hypopneic
- Operator has determined that preoxygenation with noninvasive ventilation or preoxygenation with an oxygen mask is required or contraindicated for optimal care of the patient
PRIMARY OUTCOME
- Hypoxemia during intubation, defined by an oxygen saturation of less than 85% during the interval between induction of anesthesia and 2 minutes after tracheal intubation.
SECONDARY OUTCOME
- The lowest oxygen saturation during the interval between induction of anesthesia and 2 minutes after tracheal intubation.
EXPLORATORY OUTCOME
- Hemodynamic events that could result from positive pressure or severe hypoxemia:
- Hypotension: Systolic BP< 65
- New or increased use of vasopressors
- Cardiac arrest during the interval between induction of anesthesia and 2 minutes after tracheal intubation
SAFETY OUTCOME
- These outcomes included aspiration during intubation, as reported by the operator; a new infiltrate identified on chest imaging in the 24 hours after induction; and the oxygen saturation and FiO2 at 24 hours after induction.
KEY RESULTS
A total of 1301 patients were enrolled in the trial. A total of 645 patients (49.6%) were assigned to the NIV group and 656 patients (50.4%) were assigned to the oxygen-mask group. 95.5% of the NIV group received NIV for preoxygenation. 98.8% of the oxygen-mask group received an oxygen mask for preoxygenation.
Primary Outcome
- Hypoxemia occurred in 57 of 624 patients (9.1%) in the noninvasive-ventilation group and in 118 of 637 patients (18.5%) in the oxygen-mask group (absolute risk difference -9.4 percentage points ; 95% CI, -13.2 to -5.6, P<0.001).
Secondary Outcome
- The median lowest oxygen saturation during the interval between induction of anesthesia and 2 minutes after tracheal intubation was 99% in the NIV group and 97% in the oxygen mask group (Median difference, 2%; 95% CI, 1 to 3).
Exploratory Outcomes
- An oxygen saturation of less than 80% was recorded in 39 of 624 patients (6.2%) in the NIV group and in 84 of 637 patients (13.2%) in the oxygen-mask group
- An oxygen saturation of less than 70% was recorded in 15 patients (2.4%) in the NIV group and in 36 patients (5.7%) in the oxygen-mask group
- Cardiac arrest occurred in 1 of 645 patients (0.2%) in the NIV group and in 7 of 656 patients (1.1%) in the oxygen-mask group.
- New or increased use of vasopressors occurred in 111 of 645 patients (17.2%) in the NIV group and 117 of 656 patients (17.8%) in the oxygen-mask group
- Systolic blood pressure < 65 mm Hg occurred in 18 of 621 (2.9%) of patients in the NIV group and 28 of 633 (4.4%) in the oxygen-mask group.
Safety Outcomes
- Aspiration occurred in 6 of 645 patients (0.9%) in the NIV group and in 9 of 656 patients (1.4%) in the oxygen-mask group.
- The incidence of new opacity, pneumothorax, and of oxygen saturation/FiO2 after 24 hours was similar in the two groups.
LIMITATIONS
- Overall, while 4462 patients met inclusion criteria, 3161 (70%) were excluded for various reasons, which suggests while this is a pragmatic study, the trial results still may only apply to a select group of patients.
- Notably, patients who were already receiving positive pressure ventilation at the time of eligibility assessment were excluded. These may include patients who are more at risk for hypoxemia during intubation.
- In addition, those who were deemed as high risk for aspiration were also excluded, thus limiting the information we have about the safety of NIV in this group.
- Lastly, the NIV group had a significantly higher percentage of patients who also got positive pressure ventilation (via NIV or bag mask with ventilation) during the period between induction and initiation of laryngoscopy even though both groups were allowed to do so. This is likely because of the ease of continuing the same method of preoxygenation from the assigned intervention. While it is hard to determine whether the provision of positive pressure via bag-mask device between induction and laryngoscopy also contributed to the result, the study design is pragmatic and thus has the strength of evaluating the outcomes in a real-world setting, where the original method of preoxygenation is likely carried forward during the apneic period.
EM TAKE-AWAYS
The PREOXI trial shows a clear advantage for the use of NIV for preoxygenation compared to traditional oxygen masks options. There were decreased incidents of hypoxemia and a higher median of the lowest oxygen saturation point for the NIV group. The risks of aspiration were also similar among the two groups, thus helping alleviate safety concerns around NIV. Adequately providing preoxygenation in the ED is vital given that these intubations are by definition high risk and in critically ill patients. This study should prompt us to consider the use of NIV preoxygenation for ED intubations.
REFERENCES
- Russotto V, Myatra SN, Laffey JG, et al. Intubation Practices and Adverse Peri-intubation Events in Critically Ill Patients From 29 Countries [published correction appears in JAMA. 2021 Jun 22;325(24):2507. doi: 10.1001/jama.2021.9012]. JAMA. 2021;325(12):1164-1172.
- Baillard C, Prat G, Jung B, et al. Effect of preoxygenation using non-invasive ventilation before intubation on subsequent organ failures in hypoxaemic patients: a randomised clinical trial. Br J Anaesth. 2018;120(2):361-367.
- Baillard C, Fosse JP, Sebbane M, et al. Noninvasive ventilation improves preoxygenation before intubation of hypoxic patients. Am J Respir Crit Care Med. 2006;174(2):171-177.
- Ford I, Norrie J. Pragmatic Trials. N Engl J Med. 2016;375(5):454-463.
- Gibbs KW, Semler MW, Driver BE, et al. Noninvasive Ventilation for Preoxygenation during Emergency Intubation. N Engl J Med. 2024;390(23):2165-2177.
- Weingart S. EMCrit 377 – Breaking News – The PREOXI Trial changes everything about Preoxygenation for Intubations in the Critically Ill. EMCrit. June 13, 2024.