Pulmonary artery catheters (PACs) are considered a valuable diagnostic and management tool in cardiogenic shock. For this application, their use is supported by recent evidence correlating their placement with a mortality benefit, and they are now a broadly accepted tool in cardiac critical care. In Part 1 of this 3-part Critical Care Device series, we explore the basics of the catheter.
Critical care units decreased their use of pulmonary artery catheters (PACs) following the publication of two studies showing no benefit back in 2005. So why talk about them today? Today, they are considered a valuable diagnostic and management tool in cardiogenic shock and are still routinely used in this population.1 For this application, their use is supported by recent evidence correlating their placement with a mortality benefit, and they are now a broadly accepted tool in cardiac critical care.2,3 Furthermore, time from presentation to placement seems particularly important.2,4,5 We believe the emergency clinician, and particularly the EM resident, should be familiar with PACs, and should learn to place them during residency.
In Part 1 of this series on PACs, we will explore the literature on PACs, how things have changed over the past 20 years, and provide a brief overview of how they work. In Part 2, we will cover indications for placement and the procedure of placement. Part 3 will review how to interpret and act on the data PACs generate.
Why did the PAC popularity glide downward? The two negative trials which led to substantially fewer applications of PACs were published in 2005.6,7 These trials found no difference in mortality with the use of PACs and an increased number of complications related to insertion. However, these trials either under-represented or did not include the patient populations for which PACs seem to have the greatest benefit. For instance, PAC-Man was a multicenter randomized trial that found no mortality benefit in patients admitted to an ICU who were managed with a PAC.6 In addition, 10% of patients in the PAC group experienced complications from insertion. However, only 11% of their population were patients with cardiogenic shock and this study was not powered to detect sub-group differences. The majority of studied patients had acute respiratory failure and multiorgan dysfunction (13% and 66%, respectively). Ultrasound, now the standard of care for central line placement, was not used in placement and this probably led to the high number of complications. Similarly, the ESCAPE trial examined patients with acute decompensated heart failure, but excluded those in cardiogenic shock or undergoing evaluation for mechanical support.7
In 2025, we generally would not consider placing a PA catheter in a patient with isolated septic shock, nor would we advocate for use in a stable heart failure patient simply to assess volume status. This is primarily due to the ubiquity of point-of-care-ultrasound and the two aforementioned negative trials. Nowadays, we typically reach for PACs in cases of cardiogenic shock and place them using ultrasound or under fluoroscopy. Indeed, data support their use in this way.
A recent meta-analysis of over one million patients with cardiogenic shock found a statistically significant mortality benefit of 36% compared to 47% for patients who had a PAC placed (AOR 0.71). Patients with PACs were also more likely to be on mechanical circulatory support.3 Another recent study (multicenter, retrospective, observational design) investigated whether PAC placement was associated with lower mortality in cardiogenic shock patients. This study found that placement of a PAC within the first 6 hours of presentation was correlated with only 17% mortality vs 28% with delayed or no placement, and PAC placement at any time was associated with a mortality benefit across all Society for Cardiovascular Angiography and Interventions (SCAI) stages of cardiogenic shock.4 This data comes from studies less than 10 years old and more accurately reflects modern utilization of PACs. It is our opinion that early PAC placement leads to better outcomes in certain patients and aids in management and disposition decisions. The objective data it generates and often streams into the electronic medical record (EMR) can help expedite access to definitive care and specialist involvement via resources such as mechanical circulatory support, cardiac catheterization, or shock teams.
Furthermore, the technology itself has changed considerably over time. Digital PAC data is more accurate than the older waveform data and can now stream into the EMR in real time.8 Routine ultrasound use for line placement (especially internal jugular) has improved cannulation quality and safety.9 Ultrasound is frequently used to view the distal tip of the catheter as it traverses the heart in real time, an advantage previously reserved for teams equipped with fluoroscopy.10
We hope this background dispels the frequently espoused adage that PAC "don't work" or "don't change anything," and that it also engenders some enthusiasm for why an EM resident might consider placing a PAC, even in the ED! Furthermore, an increasing number of EM residents are choosing careers in critical care, where they will routinely manage PACs. Like any diagnostic aid, applying PACs selectively and understanding how to interpret and act on the data is the key.
Where did the PAC come from? The PAC, also known as the Swan-Ganz catheter, is named after Drs. Jeremy Swan and William Ganz. They co-developed the device and first published about it in the 1970s. The catheter is a pressure-sensing, quadruple-lumen device that is inserted into a central vein via an introducer sheath, analogous to a transvenous pacing wire. The catheter ranges from 60-110 cm in length and 4-8 Fr in diameter, depending on patient characteristics and site of insertion. Similar to an arterial line, a Swan-Ganz is connected to an external pressure transducer for cardiac and vascular pressure monitoring. The catheter also features a thermodilution sensor (thermistor), which is used to measure cardiac output (CO).
The Swan-Ganz can also be used to directly measure central venous pressure/right atrial pressure (CVP/RAP), right ventricular pressure, pulmonary artery pressure (PAP), pulmonary capillary wedge pressure (PCWP), mixed venous O2 saturation (SvO2), and core body temperature. Additional hemodynamic variables, such as cardiac index, pulmonary vascular resistance, and systemic vascular resistance, can be calculated with data measured from the catheter.
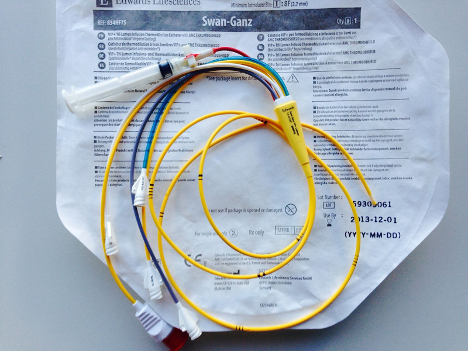
Fig. 1. Pulmonary artery catheter, Swan-Ganz (image by ICUnurses, used with permission under Creative Commons Attribution share-alike license)
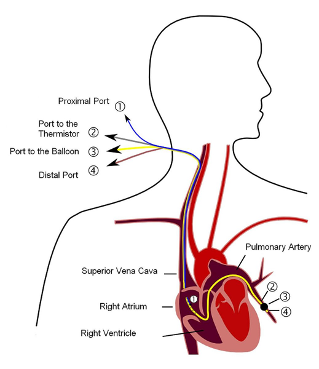
Fig. 2. Positioning of the pulmonary artery catheter (Image by Tariq Abdulla, used with Creative Commons share-alike permission)
The various lumens of the catheter terminate at different points along its length, with corresponding ports in its proximal end. To advance a Swan Ganz to its destination—typically a branch pulmonary artery—a 1-1.5 cm balloon on the distal tip is used to “float” the catheter with the direction of blood flow. The pressure tracing will help to locate the catheter position as it is advanced from the right atrium to the pulmonary artery. Fluoroscopy (or more realistically in most EDs, ultrasound) can also be used to help guide catheter placement. Once in place, each opening on the catheter will sit in a specific cardiac chamber or great vessel.
- The blue lumen is the RAP/CVP port and sits within the RA, 30 cm from the distal tip. Its functions include measuring RAP and fluid or medication administration. It is also known as the proximal injectate port, as this is where a volume of blood is injected for the thermodilution method of determining CO.
- The white lumen is 31 cm from the distal tip and also rests within the RA. It is used for infusions, hence it is also called the proximal infusion port.
- The yellow lumen is the pulmonary artery lumen or distal port. It sits slightly distal to the balloon in a branch pulmonary artery and represents the distal end of the catheter. It is connected to a pressure transducer that measures PAP or PCWP, depending on the inflation status of the balloon. When the balloon is deflated, the catheter measures blood pressure as it flows through the pulmonary circulation. As it sits within the pulmonary artery system, this measurement reflects PAP. However, when the balloon is inflated, it obstructs blood from the pulmonary artery into the microcirculation. The catheter is “wedged” in the artery. The resulting measurement distal to this occlusion is the PCWP. A blood sample drawn from this lumen can be used to measure SvO2.
- The red port is the balloon port, where air is introduced or removed to control balloon size. Its proximal end is connected to a small syringe. This port is identical to the balloon port on transvenous pacing wire.
Depending on the device model and manufacturer, a variable number of other connectors can be found in the proximal end. These typically include a thermistor connector and continuous oximetry monitoring.
Like other advanced procedural and diagnostic interpretation skills such as advanced echocardiography, repetitions and expert teaching are useful. We suggest you start now and adopt PAC use while you are still in training, and while there are expert clinicians to precept you! There is significant variability in physician abilities to accurately interpret data from a PAC.11 Our hope is that this multi-part series will help educate the EM resident on when and how to gather, interpret, and utilize this data, so that we can re-educate a generation of EM clinicians on this useful tool. In part two, we will discuss indications and the procedure of placement, and in part three we will talk about data interpretation and how to use a PAC to help management. After reading this three-part series, we challenge you to find an appropriate patient, an appropriate supervisor, and "float a Swan!"
References
- Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(17):e263-e421.
- Chow JY, Vadakken ME, Whitlock RP, et al. Pulmonary artery catheterization in patients with cardiogenic shock: a systematic review and meta-analysis. Can J Anaesth J Can Anesth. 2021;68(11):1611-1629.
- Bertaina M, Galluzzo A, Rossello X, et al. Prognostic implications of pulmonary artery catheter monitoring in patients with cardiogenic shock: A systematic review and meta-analysis of observational studies. J Crit Care. 2022;69:154024.
- Kanwar MK, Blumer V, Zhang Y, et al. Pulmonary Artery Catheter Use and Risk of In-hospital Death in Heart Failure Cardiogenic Shock. J Card Fail. 2023;29(9):1234-1244.
- Wagaman B. The efficacy of pulmonary artery catheters in reducing mortality in acute heart failure cardiogenic shock: A systematic review. Heart Lung J Crit Care. 2024;66:123-128.
- Harvey S, Harrison DA, Singer M, et al. Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-Man): a randomised controlled trial. The Lancet. 2005;366(9484):472-477.
- Binanay C, Califf RM, Hasselblad V, et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. 2005;294(13):1625-1633.
- Gracias VH, Horan AD, Kim PK, et al. Digital output volumetric pulmonary artery catheters eliminate interoperator interpretation variability and improve consistency of treatment decisions. J Am Coll Surg. 2007;204(2):209-215.
- Saugel B, Scheeren TWL, Teboul JL. Ultrasound-guided central venous catheter placement: a structured review and recommendations for clinical practice. Crit Care Lond Engl. 2017;21(1):225.
- Josan E, Pastis N, Shaman Z. Ultrasound guided pulmonary artery catheter insertion: An alternative to fluoroscopic guidance. Respir Med Case Rep. 2022;38:101678.
- Squara P, Bennett D, Perret C. Pulmonary artery catheter: does the problem lie in the users? Chest. 2002;121(6):2009-2015.



