One of our nation's greatest health challenges is the management of type 2 diabetes mellitus (T2DM). Its incidence is projected to increase by more than 50% in the next 15 years.1
Many of these diabetic patients will lack both primary and specialty physician care and will instead seek treatment for complications secondary to this disease in the ED. Despite efforts aimed at providing medications and education on the risks of T2DM, the medical community has largely been unsuccessful at curbing the number of patients with uncontrolled T2DM arriving to the ED. It is therefore critical that emergency physicians be adept at recognizing and treating the complications of this disease.
Nonketotic hyperglycemic hemichorea (NHH) is a rare manifestation of diabetes typically first seen in the ED and easily treated with prompt recognition. Despite being a rare clinical syndrome, it was reported as early as 1960. While studies have been primarily from journals of neurology, endocrinology, and radiology, description in emergency medicine literature has remained sparse.2,3,4 Therefore, we present a case of diabetic hemichorea and discuss features of its presentation and treatment relevant to the emergency clinician.
Case Report
A 66-year-old male with a past medical history of coronary artery disease, hypertension, hyperlipidemia, and T2DM presented to the ED with the complaint of one month of involuntary jerking of his right upper and lower extremities. He characterized these movements as rapid twitches that were continuously present and involved the entire limb. The patient stated that the movements progressed from his right leg to his right arm, but had not spread to his other limbs or face. His primary physician had diagnosed his symptoms as restless legs syndrome (RLS). Outpatient computed tomography (CT) scan of his head was performed due to multiple recent falls and evaluation for traumatic brain injury. He was told to seek further evaluation in our hospital after the CT scan demonstrated concern for an intracranial hemorrhage (ICH). The patient also complained of a recent mild headache but denied any visual changes, limb weakness, slurred speech, chest pain, dyspnea, or flu-like symptoms. He reported no head trauma prior to the start of these movements, history of seizures, or use of neuroleptics, steroids, or anticoagulants. The patient had been prescribed insulin lispro, however, he had not taken this for the past six months and was not performing home glucose monitoring.
On presentation the patient was mildly obese, vital signs included blood pressure of 127/66 mmHg, heart rate of 83 beats per minute, respiratory rate of 14 breaths per minute, and normal oxygen saturation with no apparent respiratory distress. Neurological examination was notable for quick, irregular, non-purposeful jerking movements and dysmetria of the right upper and lower extremities. Muscle strength and deep tendon reflexes were normal. The patient had a steady gait, good rapid alternating movements of the upper extremities, and no pronator drift. The physical examination was otherwise unremarkable.
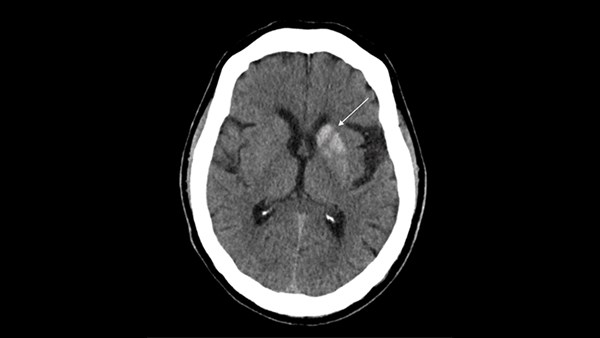
Laboratory evaluation showed elevated blood glucose (255 mg/dL), mild hyponatremia (133 mmol/L, corrected for hyperglycemia 135-137 mmol/L), chloride (95 mmol/L), and creatinine (0.57 mg/dL). Other laboratory findings were within normal limits, including potassium (4.0 mmol/L), calcium (9.6 mg/dL), urea nitrogen (15 mg/dL), CO2 (25 mmol/L), anion gap (16 mmol/L), and venous pH (7.39). A repeat CT scan of the head without intravenous (IV) contrast revealed hyperdensity of the left basal ganglia with increased attenuation throughout the left caudate nucleus and putamen, sparing of the internal capsule, and no acute hemorrhage, findings most consistent with NHH.
Neurology was consulted in the ED and recommended better glucose control to manage the patient's disease. Magnetic resonance imaging (MRI) of the brain was deferred until correction of the patient's hyperglycemia. He was treated in the ED with risperidone for choreiform movements, fioricet for headaches, IV normal saline, and a single treatment of insulin aspart for hyperglycemia.
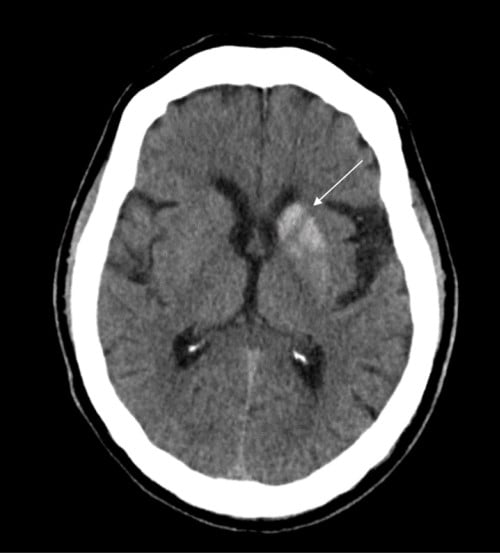
Figure 1. Axial brain CT without IV contrast showing increased attenuation of the left caudate nucleus and putamen (arrow) with sparing of the internal capsule.
Discussion
Hemichorea-hemiballismus is a rare hyperkinetic movement disorder characterized by unilateral, involuntary, and rapid jerking movements of one or both limbs.5 Basal ganglia infarction accounts for most cases, but NHH is now recognized as the second most common etiology.5 The exact pathogenesis of this disease is unknown, but is thought to entail a delayed hyperglycemic ischemia involving interrupted or depleted thalamic gamma-aminobutyric acid (GABA) input, striatal microhemorrhages, and cerebral mal-perfusion.6 The prevalence of NHH is estimated at less than 1 in 100,000, has an average age at onset of 70 years, and is described most frequently in female patients of East Asian descent.7
A number of severe conditions other than hyperglycemia can cause hemichorea-hemiballismus and should be ruled out in the ED. These etiologies include hemorrhagic and ischemic stroke, carbon monoxide poisoning, infectious diseases, neurodegenerative disorders, and neoplasms.4 Diagnosis of NHH is made by CT scan findings of unilateral hyperdensity of the putamen contralateral to the symptomatic side, with or without hyperattenuation of the caudate nucleus, and sparing of the internal capsule.8 Contrast enhancement is typically minimal or absent.8 Due to its hyperdensity on CT, the findings of NHH can be confused with other disease processes. Its unilaterality excludes microcalcification, Fahr's disease, Wilson's disease, and manganese deposition. Confinement to the basal ganglia and lack of mass effect excludes hemorrhage.5
Findings of NHH on MRI include unilateral hyperintensity on T1-weighted images without the corresponding hyperintensities on T2*-weighted images that characterize basal ganglia hemorrhage.8 Following recognition, NHH should be treated with aggressive glucose control which typically leads to resolution of the hemichorea and imaging findings.9 Refractory cases may require treatment with postsynaptic dopamine receptor antagonists such as haloperidol or risperidone.
Treatment
Prompt identification and treatment of NHH leads to excellent patient outcomes and likely resolution of symptoms. As patients with T2DM increasingly seek care in the ED, clinicians should gain familiarity with its many complications, presentations, and be prepared to distinguish them from other acute processes.
This case emphasizes the importance of including uncontrolled diabetes in the differential for new onset hemichorea-hemiballismus. It further highlights the value of diabetic patient education in the ED and outpatient follow-up in mitigating the burden of critical complications.10
Case Conclusion
The patient was provided diabetes education and discharged home following improvement of labs and symptoms. He was provided outpatient follow-up in neurology's movement disorder clinic with orders for repeat neuroimaging. Unfortunately, the patient did not return for his clinic visit and was lost to follow-up.
Take-Home Points
- The incidence of Type 2 diabetes mellitus is projected to increase by more than 50% in the next 15 years, making it important for emergency physicians to recognize and manage a range of T2DM complications.
- A number of severe conditions other than hyperglycemia can cause hemichorea-hemiballismus and should be ruled out in the ED.
- Diagnosis of NHH is made by CT scan findings.
- Prompt identification and treatment of NHH leads to excellent patient outcomes.
References
- Rowley WR, Bezold C, Arikan Y, et al. Diabetes 2030: insights from yesterday, today, and future trends. Popul Health Manag. 2017;20(10), 6-12. doi:1089/pop.2015.0181.
- Choi JY, Park JM, Kim KH, et al. Radiographic basal ganglia abnormalities secondary to nonketotic hyperglycemia with unusual clinical features. Clin Exp Emerg Med. 2016;3(4), 252-255. doi:15441/ceem.15.035.
- Lin CJ, Huang P. Delayed onset diabetic striatopathy: hemichorea-hemiballism one month after a hyperglycemic episode. Amer J of Emerg Med. 2017, 35:1036.e3-1036.e4. doi: 1016/j.ajem.2017.02.018.
- Narayanan S. Hyperglycemia-induced hemiballismus hemichorea: a case report and brief review of the literature. J Emerg Med. 2012;43(3), 422-444. doi: 1016/j.jemermed.2010.05.003.
- Cressman S, Rheinboldt M, Lin D, et al. Nonketotic hyperglycemia-induced hemichorea-hemiballism. Appl Radiol. 2018; 47(6): 24-26.
- Tan Y, Xin X, Xiao Q, et al. Hemiballism-hemichorea induced by ketotic hyperglycemia: case report with PET study and review of the literature. Transl Neurodegener. 2014;3(14). doi: 1186/2047-9158-3-14.
- Consentino C, Torres L, Nunez Y, et al. Hemichorea/hemiballism associated with hyperglycemia: report of 20 cases. Tremor Other Hyperkinet Mov. 2016;6(402). doi: 7916/D8DN454P.
- Hansford BG, Albert D, Yang E. Classic neuroimaging findings of nonketotic hyperglycemia on computed tomography and magnetic resonance imaging with absence of typical movement disorder symptoms (hemichorea-hemiballism), J Radiol Case Rep. 2013;7(8), 1–9. doi: 10.3941/jrcr.v7i8.1470.
- Lai PH, Tien RD, Chang MH et-al. Chorea-ballismus with nonketotic hyperglycemia in primary diabetes mellitus. AJNR Am J Neuroradiol. 1996;17 (6): 1057-64.
- Lewis VR, Benda N, Nassar C, et al. Successful patient diabetes education in the emergency department. Diabetes Educ. 2015;41(3), 343-350. doi: 1177/0145721715577484.