Introduction
Prehospital studies on the diagnostic potential of the FAST examination have shown little benefit as the prehospital time frame may be too early to detect significant intra-abdominal bleeding.1 However, other studies like focused echocardiographic evaluation in life support (FEEL) and prehospital application of sonography in emergencies (PHASE) show that prehospital POCUS can be successful.2,3
The FEEL trial explored physicians performing prehospital sonographic evaluations in patients with shock or in cardiac arrest and showed that ultrasound altered management in 78% of the cases.
The PHASE trial was a pilot study following paramedics successfully differentiating between cardiac activity versus standstill on ultrasound with minimal training.
Chronic obstructive pulmonary disease (COPD) and congestive heart failure (CHF) are two common comorbidities that are frequently evaluated in the prehospital setting. Both of these disease exacerbations can present with the chief complaint of dyspnea or “shortness of breath” and as such need to be differentiated diagnostically.
Our campus alone received more than 200 calls over a 12-month period regarding shortness of breath from patients with a history of either COPD or CHF. In one year, from February 2019-20, 10 prehospital cases were treated for shortness of breath, usually with nebulizer treatments, but were then found to have a CHF exacerbation after ED work-up and started on the appropriate treatment of nitrate, diuretic, or positive pressure ventilation.
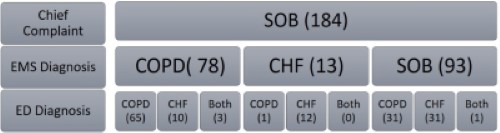
Figure 1: EMS charts from February 2019-20 with the prehospital chief complaint of SOB, an EMS-presumed diagnosis of COPD, CHF, or unknown SOB based on treatments, and an ED diagnosis of CHF, COPD, or both. The ED diagnosed 30 patients with an EMS diagnosis of SOB with a different diagnosis other than COPD, CHF, or both. Examples of these diagnoses would be “cough” or “chest pain.”
A large portion of our population has a history of both COPD and CHF, which can make the prehospital treatment of dyspnea more complicated. For example, four different prehospital cases of shortness of breath were diagnosed with both COPD and CHF exacerbations upon ED work-up.
Several studies have demonstrated the increased sensitivity and specificity of point-of-care ultrasound (POCUS) in appropriately diagnosing CHF versus COPD.5,6,7 Pulmonary edema in a CHF exacerbation can be identified on ultrasound by B-lines, a comet-tail reverberation artifact. A two-patient case series in 2010 expanded upon this knowledge and illustrated how prehospital POCUS, when used by EMS for the chief complaint of shortness of breath, could appropriately diagnose and assist in the correct management of the undifferentiated dyspneic patient.4
However, the Emergency Medical Services and Bilateral Lung Ultrasound in Emergency Medicine (EMS BLUE) study in 2017 tried to assess the feasibility of paramedics performing prehospital lung ultrasounds in medical patients in respiratory distress.8 The study fell short of meeting their thresholds for feasibility, but our resident-physician-assisted ride-along cases suggest a strong argument for its use.
Case Series: Case 1
A 52-year-old female with a past medical history of end-stage renal disease (ESRD) on peritoneal dialysis called EMS for shortness of breath over the previous three hours. The patient was a poor historian with a stated history of dialysis, but denied specific history of COPD or CHF. Per chart review later, the patient also had a history of sarcoidosis, diabetes, hypertension, and hyperlipidemia. Upon EMS arrival, the patient appeared uncomfortable, in moderate respiratory distress. She was saturating 60% on room air and was immediately placed on a non-rebreather (NRB) mask with improvement of her oxygen saturation to 100%.
Due to the patient’s poor knowledge of her medical conditions, the team performed portable ultrasound imaging of the patient’s lungs in the ambulance, which showed more than three B-lines per high-powered field anteriorly. This suggested pulmonary edema/fluid overload causing respiratory distress instead of other etiologies such as bronchoconstriction or pneumothorax.
In the ED, the patient was placed on noninvasive positive-pressure ventilation (NIPPV), and oxygen saturation remained at 100%. Her blood pressure was 184/106 mmhg. A chest X-ray revealed pleural effusions and pulmonary edema, which was likely acute volume-overload secondary to uncontrolled hypertension and renal failure on dialysis in the setting of ESRD. The patient remained stable during her ED stay with improvement of her respiratory distress and oxygenation and was admitted to the hospital for further care and monitoring.
Case Series: Case 2
A 74-year-old female with a past medical history of CHF, COPD, hypertension, and cardiomyopathy on baseline 4-liter home oxygen called EMS for shortness of breath. The patient said her symptoms developed over a period of months and worsened over the past four days, especially during ambulation to the restroom.
The patient was saturating at 94% on nasal cannula while seated and 89% with speaking and exertion. She had a blood pressure of 112/52 mmhg with a respiratory rate of 22 rpm. On physical examination, the patient surprisingly did not have wheezing, rales, or rhonchi. On further evaluation, she had no jugular venous distension or peripheral edema either. However, the patient did have pursed lip exhalation, tachypnea, and increased dyspnea from baseline. The patient endorsed taking furosemide daily and recently started spironolactone as prescribed by her primary care doctor.
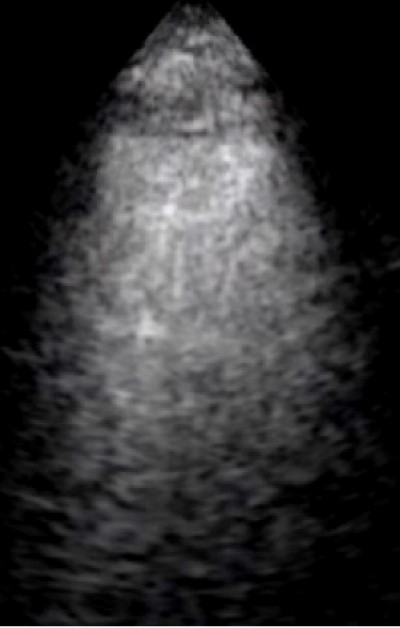
Figure 2: Portable ultrasound of lungs showing horizontal A-lines, an artifact indicating normal lung surface
Due to the unclear etiology of her symptoms, a portable ultrasound examination was performed while in the ambulance during transport to the hospital. The pulmonary examination did not show more than three B-lines per high-powered field anteriorly or laterally in bilateral lungs and showed lung sliding throughout. This suggested COPD causing respiratory distress instead of other etiologies such as CHF or pneumothorax.
In the ED, the patient received albuterol inhaler treatments with improvement of her symptoms, and her respiratory rate improved from 22 to 16 respirations per minute (rpm). The patient was evaluated with a chest computed tomography angiography (CTA) due to an elevated D-dimer, which showed no pulmonary embolism, pleural effusion, or pneumothorax. In addition, her COVID-19 test was negative. The patient was able to ambulate in the emergency department without any difficulty or hypoxia and was discharged with primary care follow-up for her COPD exacerbation.
Case Series: Case 3
A 57-year-old female with a past medical history of CHF, COPD on 2 liters home oxygen, automatic implantable cardioverter-defibrillator (AICD), diabetes mellitus, hypertension, and hyperlipidemia called EMS for shortness of breath. The patient described dyspnea symptoms for two days with increased lower extremity edema. The patient noted she was supposed to be on furosemide but had not taken it in several days. She also admitted to being intubated once in the past for similar symptoms.
The patient was saturating 99% on 2L nasal cannula with a blood pressure of 155/90 mmhg and a respiratory rate of 28 rpm with increased work of breathing. On auscultation, the patient had diffuse wheezing in all lung fields and bibasilar rales. Additionally, the patient had +2 edema in her lower extremities. Due to the wheezing on auscultation, EMS placed the patient on NIPPV and administered ipratropium/albuterol.
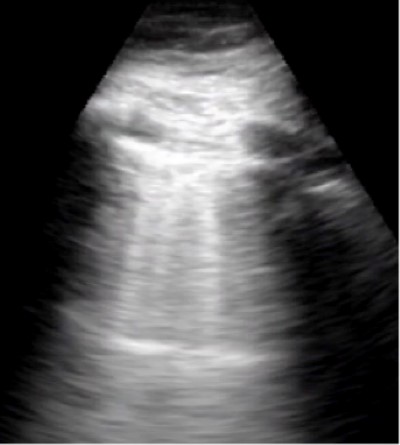
Figure 3: Portable ultrasound of lungs showing vertical B-lines, a comet-tail reverberation artifact representing the ultrasound waves interacting with an air/fluid medium, suggestive of pulmonary edema
Portable ultrasound in the ambulance during transport showed more than three B-lines per high-powered field in bilateral anterior and lateral lung fields. Portable ultrasound and physical examination suggested a multifactorial cause of patient’s dyspnea, which included both CHF and COPD exacerbations.
In the ED, the patient was transitioned from CPAP to 2L nasal cannula due to her improved work of breathing, with respiratory rate of 20 rpm and saturation of 100%. Her physical examination in the ED was documented as scattered wheezing, diminished breath sounds, and pitting lower extremity edema. Her BNP was 504 correlating with a CHF exacerbation component. Her CXR was consistent with pulmonary vascular congestion without pneumothorax. A COVID-19 swab was negative. The patient was given several medications during her ED stay, including: albuterol nebulizers, methylprednisolone, furosemide, and aspirin.
Following treatment, the patient had resolved wheezing and improved work of breathing. She was admitted to the hospital under monitored care for both COPD and CHF exacerbations.
Case Series: Case 4
A 47-year-old female with a past medical history of undifferentiated asthma vs. COPD, seizures, migraines, and breast cancer presented to her primary care physician’s office for her scheduled office visit. However, upon arrival, the patient developed worsening shortness of breath that had started earlier in the day, and EMS was called. The patient noted she had tried albuterol inhaler treatments at home with no improvement. She additionally described dyspnea with lying flat and subjective swelling throughout her body.
Upon arrival, the patient was saturating 95% on 4L nasal cannula, placed by her primary care provider, with a blood pressure of 132/104 mmhg and respiratory rate of 20 rpm with accessory muscle use. On further physical examination, she had scattered wheezing and decreased breath sounds with trace lower extremity peripheral edema. The patient was started on an ipratropium/albuterol nebulizer by EMS.
During her initial nebulizer treatment in the office, her portable ultrasound lung examination showed lung sliding in anterior, posterior, and lateral lung fields bilaterally with no B-lines visible. This suggested a bronchoconstrictive etiology over pulmonary edema.
In the ED, the patient had improved wheezing and decreased accessory muscle use. Her chest X-ray showed no obvious acute process, and her COVID-19 test was negative. She was given albuterol and methylprednisolone while in the ED. Following her treatments, she had further improvement in wheezing and was admitted for further evaluation to the pulmonary service for asthma/bronchitis exacerbation with the concern that she had needed multiple breathing treatments and had a history of intubation secondary to those symptoms.
Discussion
Shortness of breath is one of the most common EMS chief complaints, with the majority of our population endorsing a history of COPD, CHF, or both. Both disease exacerbations can present similarly with dyspnea, diminished breath sounds, tachypnea, hypoxia, and wheezing. Per our institution’s annual EMS arrivals for the chief complaint of shortness of breath, 10 patients were given nebulizer treatments, the appropriate management for COPD exacerbations, but then diagnosed with a CHF exacerbation after ED work-up.
An additional 10 patients with a history of COPD and CHF over the past year were started on oxygen prehospital, when further data about their pulmonary status, such as pulmonary edema, if obtained in the prehospital setting, could have expedited or narrowed ED workup and treatment.
It is important to keep in mind that data was pulled from an initial EMS complaint of shortness of breath with the ED diagnosis of COPD, CHF, or both. This excludes patients who had a different ED diagnosis such as asthma, fluid overload, shortness of breath, etc.
Pharmaceutical interventions have both risks and benefits. Nebulizer treatments may worsen tachycardia, and steroids may make blood sugar management more challenging. Failure to intervene may result in intubation in a population with a high risk and difficult airways. These cases strongly suggest that if prehospital systems were equipped with point-of-care ultrasound, it would aid in the diagnosis of the dyspneic patient, especially in those with a history of CHF or fluid overload of other etiology and COPD or lung parenchyma disease, as POCUS can visualize B-lines suggestive of pulmonary edema.
Prehospital POCUS is quick, non-invasive, and effective. It would have little extra cost to the patient and may be financially beneficial to the patient and healthcare system by decreasing nebulized treatments in the dyspneic patient when the patient instead would benefit from shifting and diuresing fluid. This prehospital diagnostic guide could have even further triaging capabilities and applications in fluid-overloaded patients — dialysis patients, for example — who are confined to their homes during floods or natural disasters. This application could assess pulmonary fluid status to aid in a decision of whether or not to transport a patient via helicopter from home, or wait an additional day or two for water levels to recede during times of natural disasters. For all of these reasons, real-time evaluation with POCUS of the acutely dyspneic patient can be an invaluable prehospital tool.
Importance of Awareness
In Case 1, the patient was accurately and effectively diagnosed with fluid overload with the utilization of ultrasound. This expedited her care on arrival to the ED and allowed for the correct medication administration and avoided other unnecessary treatments that target bronchoconstrictive etiologies.
In Case 2, it is important to note that during prehospital transport, the patient was not able to receive breathing treatments, as she did not meet the physical examination criteria — including wheezing — per regional paramedic protocols to start such interventions. Her portable ultrasound examination placed COPD as a more likely etiology of her symptoms and could have expedited her care and even decreased her ED disposition time. It is important to consider not only the impact prehospital ultrasound can have on patient care, but also the effect it can have on rethinking and restructuring prehospital treatment protocols.
In Case 3, this patient’s bedside ultrasound in the ED showed B-lines bilaterally, a plethoric IVC, and a decreased cardiac ejection fraction. Prehospital ultrasound showing B-lines alerted ED staff immediately on arrival that the cause of the patient’s symptoms was multifactorial and expedited her care.
In Case 4, the patient’s prehospital ultrasound examination placed CHF and pneumothorax lower on the differential. Following effective communication between prehospital and ED teams, the patient received only one nebulizer treatment, a COVID-19 test, and a chest X-ray. Unnecessary laboratory testing such as BNP or other invasive testing was avoided due to prehospital imaging and effective communication.
The utilization of ultrasound in the prehospital setting has potential benefits with limited downside. This case series demonstrates the different benefits of prehospital POCUS performed by our clinicians during a ride-along. However, we were limited in having resident physicians acquiring and interpreting these images. We aim to further explore the utility of training our EMS clinicians in the use of POCUS, and we hope that prehospital image acquisition can lead to earlier diagnosis and shorten time to appropriate medical intervention.
Disclaimer: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Roantree RAG. EMS ultrasound use. StatPearls [Internet]. Published Sept. 19, 2020. Accessed Oct. 12, 2020.
- Breitkreutz R, Walcher F, Ilper H, et al. Focused echocardiography in life support: The subcostal window. European Journal of Trauma and Emergency Surgery. 2009;35(4):347-356.
- Rooney KP, Lahham S, Lahham S, et al. Pre-hospital assessment with ultrasound in emergencies: Implementation in the field. World Journal of Emergency Medicine. 2016;7(2):117.
- Zechner PM, Aichinger G, Rigaud M, Wildner G, Prause G. Prehospital lung ultrasound in the distinction between pulmonary edema and exacerbation of chronic obstructive pulmonary disease. The American Journal of Emergency Medicine. 2010;28(3).
- Koh Y, Chua MT, Ho WH, Lee C, Chan GW, Sen Kuan W. Assessment of DYSPNEIC patients in the emergency department using point-of-care lung and cardiac ultrasonography—a prospective observational study. Journal of Thoracic Disease. 2018;10(11):6221-6229.
- Guttikonda SN, Vadapalli K. Approach to undifferentiated dyspnea in emergency department: AIDS in rapid clinical decision-making. International Journal of Emergency Medicine. 2018;11(1).
- Schick M. Breathing easier with point-of-care ultrasound. EMRA/EM Resident. Published Jan. 9, 2015. Accessed Oct. 13, 2021.
- Becker TK, Martin-Gill C, Callaway CW, Guyette FX, Schott C. 398 emergency medical services and bilateral lung ultrasound in emergency (EMS Blue) study. Annals of Emergency Medicine. 2017;70(4).



