Term: Type of Error
Type I and Type II Errors and alpha and beta. These terms are basic for statisticians, but they can be quite confusing to those who do not use them frequently. Am I not not rejecting the null hypothesis? Is that a triple negative?
There is a high likelihood that a question related to this topic will appear on your board exams. The chart below hopefully provides a helpful guide for the non-researcher.
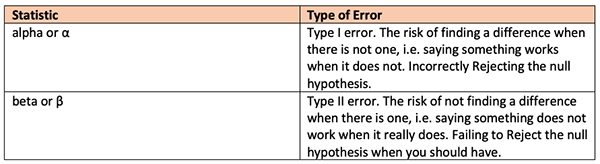
Note that “alpha” or “α” is used to signify the risk of a Type I error allowed by researchers. The actual chance that this error would occur is what we are familiar with as a “p value." A “p value” is a result after the study is done, while α is determined by the researchers prior to any data collection.
“Beta” or “β” is the risk of a type 2 error. It is related to the “power” of a study such that 1- β = power.
Typical numbers accepted in medical studies are α of 0.05 and β of 0.2. We generally accept not finding that something works when it actually might slightly more than saying something works when it doesn’t. Several factors contribute to this in practice, but the most important one is sample size, or the number of patients in a study.



