While in the ED, especially as we deal with high national rates of ICU boarding, you must be ready to recognize subarachnoid hemorrhage and deliver high-level neurocritical care to these patients.
Case
A 52-year-old male presents to the ED with a sudden onset headache. He states it’s “all over” his head and radiates to his neck, and it began 7 hours ago. He denies this occurring before. His vitals on arrival are as follows: HR 97, BP 175/96, RR 12, Temperature 37ºC.
Introduction
Subarachnoid hemorrhage (SAH) is a terrifying, relatively frequent cause of intracranial bleeding. It has a significant degree of morbidity and mortality. SAH makes up ~10% of all strokes. SAH can be aneurysmal (more common) or non-aneurysmal. The average age of aneurysmal rupture causing SAH is ~50 years old.1
An aneurysm is a protrusion from an artery that is prone to rupture. Saccular (berry) aneurysms have a thin-walled tunica media that protrudes from an artery due to a defected elastic lamina. Fusiform aneurysms are a circumferential dilatation of the entire section of a particular artery.2 Non-aneurysmal causes of SAH include trauma, arterio-venous malformation, vasculitis, amyloidosis, bleeding diathesis, or sympathomimetic abuse.3
The two biggest risks for aneurysm formation and subsequent SAH are smoking and hypertension. Family history of specific traits, such as Ehlers-Danlos and polycystic kidney disease are risks as well, although the majority of SAH are nongenetic.4,5
As disturbing as they are to patients, most cerebral aneurysms do not rupture. Their lifetime prevalence is about 3% in the U.S. alone.6 About 85% of them are located in the anterior circulation, mainly on the Circle of Willis; 20-30% of patients have multiple aneurysms.2
Sometimes unruptured aneurysms can cause headaches. These headaches often mimic SAH. There is no major consensus on which aneurysms need to be managed surgically. It is agreed that aneurysms >7 mm grow faster and have a higher rate of rupture (eg, 5-year rupture rates for 7-12 mm risk was 2.5%; 13-24 mm, 14.5%).7
We prefer to stay out of this debate (for obvious reasons). However, here’s a summary where there is decent support for intervention: symptomatic unruptured aneurysms of all sizes, asymptomatic aneurysms >7-10 mm, remaining aneurysms of all sizes in those with SAH.
Triggers of rupture are not always identifiable. Some can occur during sleep.2 There is often some element of exertional event tied with many other confounding variables including consuming caffeine, sexual intercourse, or competitive athletics.
The pathophysiology of SAH includes rupture of the vessel, resulting in blood rapidly entering the subarachnoid space. There is a subsequent increase in intracranial pressure with symptoms of an intense headache.
Presentation
The most common presenting symptom is a sudden, severe headache, found in basically all cases (97%).8 The headache is nonspecific and intense, with nausea and vomiting in about 77% of patients. Loss of consciousness is seen in half of patients.
Seizures are uncommon, (~10% of patients), but they are arguably the most concerning symptom if present early on.9
Sudden death due to SAH occurs in about 10-15% of patients.
EKG can show nonspecific changes suggestive of ischemia, such as ST depression, QT prolongation, or deep T wave inversions.10
The Ottawa Subarachnoid Hemorrhage Rule may be used in neurologically intact patients presenting with acute, nontraumatic headaches that reach max intensity within one hour. The clinical decision rule is 100% sensitive, with a specificity of 15%. Clearly this is a one-sided rule, and caution should be noted when a positive rule is obtained.11
Diagnosis
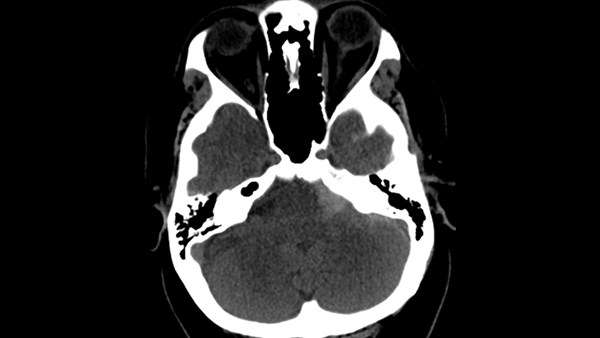
The first test is always a CT head without contrast. Blood is found in the subarachnoid space 92% of the time if scan is performed within 24 hours. If the CT head scan is done in <6 hours, the sensitivity is virtually 100%.12 Therefore, if a patient’s symptoms truly began <6 hours prior and the CT scan is negative, SAH workup is complete and no further diagnostic workup is warranted.
The critical caveats we must mention: 1) the CT scan is reviewed by expert radiologists, 2) the CT scanner is a modern model (multidetector CT).13
Lumbar puncture should be performed if there is negative head CT and patient presentation is >6 hours with a concerning story.14 Sensitivity is 100% and NPV 100% with a specificity of 65%. Classic findings from the LP include an elevated opening pressure but is not always reliably present. RBC count can be >2000 in CSF.15 Classically, the elevated RBC count does not decrease from tubes 14. If the RBC count does diminish, this still does not mean it was a “traumatic tap”! Always have a high index of suspicion!
Xanthochromia (yellow tint from Hgb breakdown) is the most specific finding and in the setting of a severe headache is virtually diagnostic of SAH. If unsure, compare the vial of CSF to a vial of tap water against a white background. Xanthochromia is rarely found <2 hours after symptom onset.16
The absence of RBCs in the final LP tube and absence of xanthochromia >2 hours after symptom onset rules out SAH with a sensitivity of 100%.
Aside from SAH, Xanthochromia can be seen in hyperbilirubinemia >10 mg/dL, and severely traumatic taps where there is typically >100k RBCs.
CTA and MRA can both equally detect aneurysms ~3-5 mm or larger. New, multidetector CTA has improved to >97% sensitive/specific for SAH. In many institutions CTA has been seen as a reasonable alternative to LP (the main drawbacks of CTA are cost and radiation). LP is thousands of dollars cheaper however can be painful, time-consuming, and not without risk. Post-LP headaches are a rare but a substantial source of patient suffering. In short, the traditional approach is still CT, followed by LP if there is concern for SAH and the patient presents >6 hours form symptom onset. Board exams want you to choose LP over CTA.17
Management
Once diagnosed, consultation with a neurosurgeon should be immediate.
All SAH patients should be admitted to an ICU. Definitive management includes surgical clipping and endovascular coiling, but many patients will require critical care in the ED. Nearly 35% of these patients have worsening neurological functioning after admission.18
While in the ED, especially as we deal with high national rates of ICU boarding, you must be ready to deliver high-level neurocritical care to these patients.
Be ready to intubate many of these patients, especially those with a declining GCS. Intubation also allows for better critical care monitoring in the setting of elevated ICP, hemodynamic instability, and/or need for heavy sedation/paralysis.19
When intubating, hypoxia and hypotension are associated with increased mortality in brain injured patients, so care must be taken to avoid these complications during intubation.20 The goal is to prevent sympathetic reflex response to intubation, therefore perform the usual optimization techniques you would normally do for first-pass success in intubation, with extra attention to preventing rapid shifts in BP, heart rate, and oxygenation status. Consider using fentanyl 3-5 mcg/kg to prevent reflex sympathetic responses <5 minutes prior to intubation, but literature is lacking. Ketamine, etomidate, or propofol are fine induction agents. Rocuronium is preferred over succinylcholine, as the latter may increase ICP and fasciculations which may cause oxygen consumption in those with brain injury.
In terms of blood pressure control, guidelines are controversial. A goal of SBP <160 is reasonable. Labetalol, nicardipine, clevidipine are preferred agents. The benefit of lowering blood pressure might be offset by the risk of infarction (remember, CPP = MAP – ICP). If ICP is high, then the only variable maintaining perfusion is MAP. The patient’s consciousness might be a helpful marker: alert and oriented, aim for SBP ~140. If GCS is impaired, aim for SBP ~160.21-23 Arterial lines can be very helpful in optimizing BP range.
Seizure prophylaxis is widely debated with no consensus. It is agreed that those who do seize should be started on an anticonvulsant agent.24
While in the ED, monitor for electrolyte changes and aggressively correct abnormalities. Fever and hyperglycemia are associated with poorer outcomes.25
Consider ventriculostomy for direct ICP monitoring if enlarged ventricles, consider craniectomy in select patients. This discussion should be held with neurosurgery.
Bottom line: any change in clinical status warrants a stat CT head scan!
Complications
Overall, SAH has a high mortality rate, with the average being around 30%. The majority die within 30 days.26
Perhaps the most well-known and feared complication is cerebral vasospasm, which is a form of delayed cerebral ischemia. The pathophysiology is unclear, but it is a form of inflammatory complication where lysis of clots and endothelial damage cause smooth muscle contraction. It is associated with poor neurologic decline and high mortality. The complication is not seen in the ED, as it presents with neurological decline nearly 3 days later. In order to prevent this, all patients should receive nimodipine as early as possible.27 Nimodipine can be started in the ED early in the patient’s disease course.
Other complications include rebleeding, with the highest risk being in the first 24 hours. Hydrocephalus is caused by obstruction of the CSF by blood products. It is both an early and late complication, so neurosurgery will consider drain placement. Hyponatremia is common and likely due to hypothalamic injury. Target euvolemia and normal electrolyte balance. Isotonic saline is the crystalloid of choice (this is one of the few times we support the use of normal saline as the preferred crystalloid!).
Prognosis
The most important prognostic factors for good outcome are as follows: consciousness and neurologic exam on initial evaluation, younger age, and the amount of blood on initial CT. Long term complications for survivors include neurocognitive disability, epilepsy, and lasting focal deficits. Unfortunately, those with SAH have a small risk of recurrence of SAH, despite successful repair. Family members have a five-fold risk of SAH compared to the rest of the population.
References
- Shea AM, Reed SD, Curtis LH, et al. Characteristics of nontraumatic subarachnoid hemorrhage in the United States in 2003. Neurosurgery 2007; 61:1131.
- Stehbens WE. Aneurysms and anatomical variation of cerebral arteries. Arch Pathol 1963; 75:45.
- Austin G, Fisher S, Dickson D, et al. The significance of the extracellular matrix in intracranial aneurysms. Ann Clin Lab Sci 1993; 23:97.
- Vlak MH, Rinkel GJ, Greebe P, et al. Lifetime risks for aneurysmal subarachnoid haemorrhage: multivariable risk stratification. J Neurol Neurosurg Psychiatry 2013; 84:619.
- Korja M, Silventoinen K, McCarron P, et al. Genetic epidemiology of spontaneous subarachnoid hemorrhage: Nordic Twin Study. Stroke 2010; 41:2458.
- Etminan N, Rinkel GJ. Unruptured intracranial aneurysms: development, rupture and preventive management. Nat Rev Neurol 2016; 12:699.
- Johnston SC, Wilson CB, Halbach VV, et al. Endovascular and surgical treatment of unruptured cerebral aneurysms: comparison of risks. Ann Neurol 2000; 48:11.
- Edlow JA, Caplan LR. Avoiding pitfalls in the diagnosis of subarachnoid hemorrhage. N Engl J Med 2000; 342:29.
- Butzkueven H, Evans AH, Pitman A, et al. Onset seizures independently predict poor outcome after subarachnoid hemorrhage. Neurology 2000; 55:1315.
- Junttila E, Vaara M, Koskenkari J, et al. Repolarization abnormalities in patients with subarachnoid and intracerebral hemorrhage: predisposing factors and association with outcome. Anesth Analg 2013; 116:190.
- Bellolio MF, Hess EP, Gilani WI, et al. External validation of the Ottawa subarachnoid hemorrhage clinical decision rule in patients with acute headache. Am J Emerg Med 2015; 33:244.
- Kassell NF, Torner JC, Haley EC Jr, et al. The International Cooperative Study on the Timing of Aneurysm Surgery. Part 1: Overall management results. J Neurosurg 1990; 73:18.
- Perry JJ, Spacek A, Forbes M, et al. Is the combination of negative computed tomography result and negative lumbar puncture result sufficient to rule out subarachnoid hemorrhage? Ann Emerg Med 2008; 51:707.
- Vermeulen M, van Gijn J. The diagnosis of subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry 1990; 53:365.
- Czuczman AD, Thomas LE, Boulanger AB, et al. Interpreting red blood cells in lumbar puncture: distinguishing true subarachnoid hemorrhage from traumatic tap. Acad Emerg Med 2013; 20:247.
- Wijdicks EF, Kallmes DF, Manno EM, et al. Subarachnoid hemorrhage: neurointensive care and aneurysm repair. Mayo Clin Proc 2005; 80:550.
- McCormack RF, Hutson A. Can computed tomography angiography of the brain replace lumbar puncture in the evaluation of acute-onset headache after a negative noncontrast cranial computed tomography scan? Acad Emerg Med 2010; 17:444.
- Helbok R, Kurtz P, Vibbert M, et al. Early neurological deterioration after subarachnoid haemorrhage: risk factors and impact on outcome. J Neurol Neurosurg Psychiatry 2013; 84:266.
- Mayberg MR, Batjer HH, Dacey R, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage. A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke 1994; 25:2315.
- Perkins ZB, Wittenberg MD, Nevin D, Lockey DJ, O'Brien B. The relationship between head injury severity and hemodynamic response to tracheal intubation. J Trauma Acute Care Surg. 2013 Apr;74(4):1074-80. doi: 10.1097/TA.0b013e3182827305. PMID: 23511147.
- Schmidt JM, Ko SB, Helbok R, et al. Cerebral perfusion pressure thresholds for brain tissue hypoxia and metabolic crisis after poor-grade subarachnoid hemorrhage. Stroke 2011; 42:1351.
- Wijdicks EF, Vermeulen M, Murray GD, et al. The effects of treating hypertension following aneurysmal subarachnoid hemorrhage. Clin Neurol Neurosurg 1990; 92:111.
- Naval NS, Stevens RD, Mirski MA, Bhardwaj A. Controversies in the management of aneurysmal subarachnoid hemorrhage. Crit Care Med 2006; 34:511.
- Marigold R, Günther A, Tiwari D, Kwan J. Antiepileptic drugs for the primary and secondary prevention of seizures after subarachnoid haemorrhage. Cochrane Database Syst Rev 2013; :CD008710.
- Kruyt ND, Biessels GJ, DeVries JH, et al. Hyperglycemia in aneurysmal subarachnoid hemorrhage: a potentially modifiable risk factor for poor outcome. J Cereb Blood Flow Metab. 2010;30(9):1577-1587. doi:10.1038/jcbfm.2010.102
- Barker FG 2nd, Ogilvy CS. Efficacy of prophylactic nimodipine for delayed ischemic deficit after subarachnoid hemorrhage: a metaanalysis. J Neurosurg 1996; 84:405.
- Mackey J, Khoury JC, Alwell K, et al. Stable incidence but declining case-fatality rates of subarachnoid hemorrhage in a population. Neurology 2016; 87:2192.