James Hall, MD, Univ. of Missouri-Kansas City, Kansas City, MO
Sajid Khan, MD, Clinical Assistant Professor, Dept. of Emergency Medicine, Univ. of Missouri-Kansas City, Kansas City, MO
Case Report
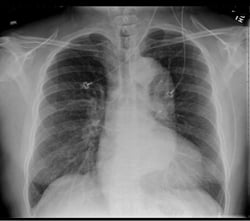
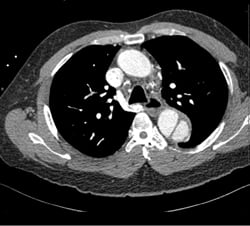
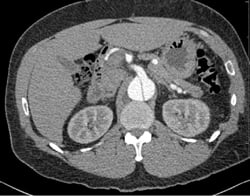
A 49-year-old male presents with a chief complaint of blurred vision. His vital signs show a blood pressure of 215/159 mmHg, a heart rate of 98, and respiratory rate of 18. Physical examination is unremarkable except for bilateral papilledema. His past medical history includes hypertension, which is poorly controlled due to medication non-compliance. A chest x-ray reveals a mediastinum measuring 11 cm. (Image 1) Based on this finding, the patient is immediately started on an esmolol drip, and a high-resolution CT angiogram of the chest is obtained. The CT reveals a Stanford type B, DeBakey type IIIb descending aortic dissection from immediately distal to the origin of the left subclavian artery to the origins of the renal arteries, with the celiac trunk being supplied by the false lumen. (Images 2 and 3) After the patient's heart rate is controlled on esmolol, a nitroglycerin drip is started to provide pressure control. He is ultimately admitted to the ICU for medical management, and discharged four days later.
Discussion
Estimates of the overall incidence of aortic dissection range between 0.5 and 10 cases per 100,000.1-3 Thoracic aortic dissections are subdivided based upon the region of the aorta that is involved in the dissection. Stanford classification separates them into type A — those that have involvement of the ascending aorta (but may also have involvement of the descending segment), and type B — those which are confined to the descending aorta only. DeBakey classification divides them into 3 classes: type I, if both ascending and descending elements are present, type II if there is only ascending involvement, and type III with only descending involvement. Type III dissections are further subdivided into types IIIa and IIIb depending on their location relative to the diaphragm. The ascending variety is more severe as it can be complicated by vascular compromise of the great vessels of the head and upper extremities and disruption of the annular ring of the aortic valve, resulting in acute aortic insufficiency and heart failure.
The classic presentation of sudden-onset, tearing chest pain is the most common by far; however, evidence of heart failure, shock, vascular compromise, focal neurologic deficits, or aortic valvular dysfunction may be the presenting signs and symptoms.3,4 Interestingly, 10-15% of all dissections present with no chest pain, and pulse deficits are present in only about 15% of patients.3,5 There are multiple case reports of patients with vague symptoms, such as anorexia and night sweats, who were ultimately found to have chronic dissections and no other cause for their symptomatology.3 Atypical and vague complaints account for many cases being missed initially.
While dissection can be spontaneous, the vast majority of patients will have one or multiple risk factors. The greatest risk factor for development of an aortic dissection is hypertension. Other risk factors include the connective tissue disorders Ehlers-Danlos and Marfan syndrome, any family history of aneurysms, recent blunt trauma or deceleration injury, a bicuspid aortic valve, and pregnancy.1,3, 6
Evaluation for aortic dissection often begins with an ECG and a chest x-ray, as these bedside tests are generally readily available. These two tests can help to rapidly risk-stratify patients with acute chest pain as potential aortic dissections, and help to promote proper treatment and avoid adverse events. If a proximal dissection involves any of the coronary ostia, an ECG may shows signs consistent with an acute myocardial infarction; however, as many as one-third of initial ECGs may be normal.5 Chest x-rays offer a sensitivity of approximately 65% for aortic disease,6, 7 and so are not perfect tests; clinical suspicion should also play into management.
As many as one in eight chest x-rays will be completely normal in acute dissection.5 The prototypical finding is a widened mediastinum, usually greater than 8 mm, though other abnormal findings seen in dissection include intimal calcium separation (extension of aortic shadow >5mm beyond the aortic wall), and a left-sided pleural effusion.
Given the severity of the disease and the relatively high incidence of diagnostic uncertainty or ambiguous data, additional imaging should be pursued in cases of suspected aortic dissection.8 There are multiple additional imaging modalities available, including CT, MRI, and transesophageal echocardiogram (TEE), as well as traditional angiography. The decision is often guided by utility and convenience; thus, CT angiogram has become the modality of choice. Patient factors may also guide advanced imaging, and TEE may well be the test of choice in unstable patients as it can be done at the bedside without risk of the patient leaving the emergency department for imaging. Pregnancy, contrast allergies, ability to lie flat, and patient size may also guide imaging decisions.
Initial goals of management should focus on heart rate and hypertension. Higher heart rates increase the frequency of maximum stress on the dissection, and higher mean arterial pressures lead to increased average stress on the damaged vessel. Oftentimes patients with acute aortic dissection will present with severely elevated blood pressure, and it can be tempting to try and quickly lower their pressure with nitroprusside or other agents. It should be remembered, however, that anti-hypertensive medications that reduce blood pressure by reducing overall systemic vascular resistance will likely result in a compensatory hyperdynamic cardiac response that increases the heart rate and can worsen the disease process. Therefore, initial management of aortic dissection first requires beta-blockade to blunt this response. Esmolol is typically the agent of choice since it is easily titrateable and has a short duration of action; alone, it will have minimal to no effect on overall pressure. Once the heart rate approaches 60 bpm, the target for systolic blood pressure should be between 100 and 120 mmHg;9 a second agent, typically a nitrate-based vasodilator, should be added after beta-blockade if needed.
Mortality is much higher in dissection involving the ascending segment, and the standard of care has become endovascular stenting. Mortality for patients undergoing surgical intervention is near 30%, while those who undergo no invasive procedure have a mortality of about 60%.10 The standard of care for descending (Stanford type B) dissections is long-term medical management through control of hypertension and other risk factors. Many of these patients have refractory hypertension, requiring on average four medications to control their blood pressure.10 Unless associated with a rapidly expanding aneurysm, patients with descending dissections are not typically managed surgically. This has been supported by the INSTEAD trial (INvestigation of STEnt-grafts in Aortic Dissection), which showed no difference in two-year mortality between those with descending stents and those treated medically.11
While acute thoracic aortic dissection can represent a source of major morbidity and mortality, prompt recognition in the emergency department can improve patient outcomes. We should be vigilant, as diagnosis can sometimes be difficult, and identifying high-risk patients may uncover occult disease. Once discovered, proper control of heart rate and blood pressure are the mainstays of treatment. Whether treated surgically or medically, it is our responsibility to be the first to combat dissections.