Knowing when to stop volume resuscitation in the unstable shock patient is a question that plagues both the emergency physician and the critical care doctor.
This question, in part, has been studied in large randomized controlled trials and discussed for decades.1-4 An emerging mental model proposes that ideally we should be assessing both sides of the heart. Using ultrasound measurements such as velocity time integral we can look to see if cardiac output increases after a 500 mL bolus or a passive leg raise. This answers "Can the left side use it?" The other half of the question asks "Can the right side take it?" There have been many studies and articles about the use of central venous pressures (CVP) to answer if the right ventricle can handle additional volume, and for more than a decade, the evidence has mounted that CVP is poorly suited to answer this question.5 Previously, CVP was the best we had in point of care ultrasound to assess right ventricle fluid tolerance, but VExUS was designed to succeed where CVP has failed.
To perform the study, you will need an ultrasound machine capable of pulse wave Doppler, and preferably a curvilinear probe as the structures to be imaged are deep anatomically. The patient should be supine with the head of the bed parallel to the ground (head of bed down).6 To start the study, first image the inferior vena cava (IVC), about 2 cm caudal to the entry of the hepatic veins as this has been shown to be the most sensitive location.7 This should be an intrahepatic portion of the IVC. If the diameter is less than 2 cm then you can stop, and VExUS would suggest that significant venous congestion is not present.8
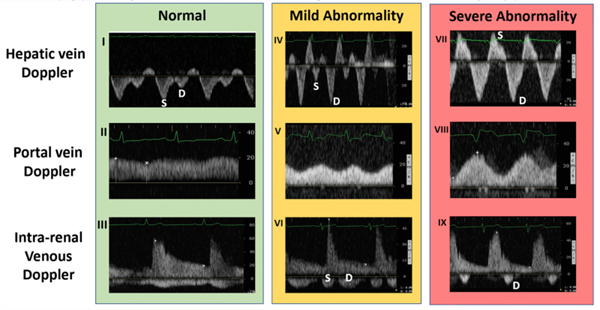
If the IVC is greater than 2 cm in diameter, then you proceed with the rest of the protocol. Find one of the hepatic veins by scanning in the anterior right upper quadrant, and any of the three hepatic veins (right, middle, or left) will suffice. The hepatic veins are the only large vessels within the liver that meet up with IVC.9 Once you have found one of the hepatic veins, first check that flow is away from the probe by changing to color flow and looking for blue flow. Then place the pulse wave gate within the hepatic vein where it meets the IVC to produce a waveform. A physiologic waveform consists of two predominant waves. The first, and larger wave, is the systolic wave (S-wave). The second wave is the diastolic wave (D-wave). A mildly abnormal hepatic vein portion of the study is when the D-wave is found to be larger than the S-wave. A severely abnormal hepatic vein portion of the study is reversal of flow of the S-wave (see figure 3).8
Figure 1. Hepatic vein Doppler with severe abnormality grade tracing 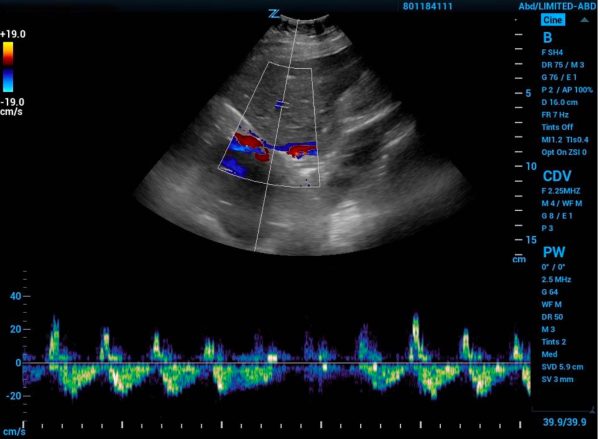
Next, you need to find the portal vein. Place the probe in the right middle axillary line over the liver and scan towards the posterior axillary line until the portal vein comes into view. Using color flow assess that flow is towards the probe and red on the screen. Next place the pulse wave doppler gate within the portal vein to produce a waveform. A normal physiologic tracing has minimal variability. Variability is defined by: Pulsatility Index = (Vmax – Vmin)/Vmax. Minimal variability is a pulsatility index <30%. As this pulsatility increases the results are more abnormal. Mild abnormalities in this portion of the study are a pulsatility index of 30%-49% and severe abnormality is >50%.8
Figure 2. Portal vein doppler with normal grade tracing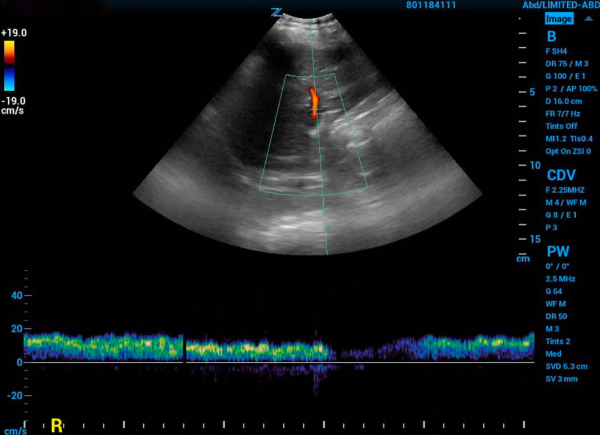
The last, and typically felt to be the most difficult component of this exam, is intrarenal vein doppler. To do this you can use either kidney. Again use color Doppler to identify the intrarenal vessels and target the largest vein (blue flow as venous flow should be predominantly medial) you can find. Place the pulse wave doppler gate within the vessel. The waveform will likely reflect both venous and arterial flow given the close anatomic relationship of the vessel in the kidney but we will focus on the negative portion of the tracing (again, flow away from the probe and venous here). Normal intrarenal venous flow is constant and near monophasic. Mildly abnormal tracings show interruptions in flow and a systolic and diastolic component of the tracing. Severely abnormal findings only show diastolic flow tracing. 8
Figure 3. Ultrasound findings by location and progressive abnormality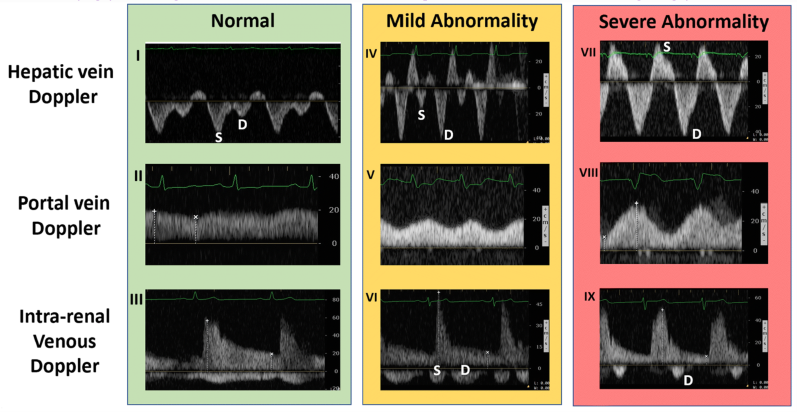
Once you have the components above you can interpret them with the VExUS scoring system and assign a grade that correlates with a qualitative degree of venous congestion.
Table 1. Grading of VExUS
|
Grade |
Ultrasound findings |
Degree of congestion |
|
0 |
IVC <2 cm |
No significant congestion |
|
1 |
IVC >2 cm with any combination of normal or mildly abnormal findings |
Mild congestion |
|
2 |
IVC >2 cm and only one severely abnormal finding |
Moderate congestion |
|
3 |
IVC >2 cm and two or more severely abnormal findings |
Severe congestion |
The two VExUS studies, first the observational study and then the validation study, both showed a strong correlation with the VExUS grading system and subsequent development of acute kidney injury later during their stay.6,8 Using VExUS to know when to stop volume resuscitation may help us to avoid congestion-related organ dysfunction.
VExUS does not prescribe an algorithm to use. It does not mandate that volume resuscitation be discontinued at grade 1 or 2 or even 3. Additionally, the protocol is somewhat time-intensive and may require a decent amount of practice to master. Also, improvements in outcomes with the use of VExUS are at this point theoretical and many similar ideas (such as ultrasound estimation of CVP) have later failed to provide clinically useful information.
Despite these drawbacks, VExUS makes a lot of sense conceptually. A distended and rigid IVC does transmit pressures from the right ventricle in reverse given that blood acts like a near-incompressible column.7
VExUS does not replace a full exam, reasoned clinical judgment, or a broader clinical picture, but it does provide a non-invasive, point-of-care, theoretically sound approach to the question of "can the right side take it?"
References
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368-1377.
- The ProCESS Investigators. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683-1693.
- The ARISE Investigators and the ANZICS Clinical Trials Group. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371:1496-1506.
- Mouncey PR, Osborn TM, Power GS, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372:1301-1311.
- Marik PE, Baram M, Vahid B. Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest. 2008;134(1):172-8.
- Beaubien-Souligny W, Benkreira A, Robillard P, et al. Alterations in Portal Vein Flow and Intrarenal Venous Flow Are Associated With Acute Kidney Injury After Cardiac Surgery: A Prospective Observational Cohort Study. J Am Heart Assoc. 2018;7(19):e009961.
- Wallace DJ, Allison M, Stone MB. Inferior vena cava percentage collapse during respiration is affected by the sampling location: an ultrasound study in healthy volunteers. Acad Emerg Med. 2010;17:96–99.
- Beaubien-Souligny W, Rola P, Haycock K, et al. Quantifying systemic congestion with Point-Of-Care ultrasound: development of the venous excess ultrasound grading system. Ultrasound J. 2020;12:16.
- Desser TS, Sze DY, Jeffrey RB. Imaging and Intervention in the Hepatic Veins. Am J Roent. 2003;180:1583-1591.