A 34-year-old previously healthy female presents with abrupt onset right-sided headache, vertigo, and vomiting associated with diplopia and perioral paresthesias. On exam, the patient is afebrile with normal vital signs. Her neurologic exam is remarkable for a unilateral lateral gaze palsy, multidirectional nystagmus, and unsteadiness of gait. She exhibits persistent retching despite antiemetics.
What is on your differential? What labs or imaging studies would you pursue at this point?
Background
Acute onset headache, vertigo, and ophthalmoplegia should invoke a broad differential diagnosis from the emergency physician that considers multiple critical and emergent diagnoses.1 This would include an evaluation for hemorrhagic, ischemic, and vascular cerebrovascular accidents because these are highly morbid, critical diagnoses with time-sensitive treatments. Numerous stroke mimics such as hypoglycemia, seizure with postictal deficits, multiple sclerosis, intracranial tumor, toxic-metabolic disturbances, meningoencephalitis, Wernicke's encephalopathy, and extrapyramidal symptoms must also be considered. For now, let's focus on three sinister syndromes causing acute headache, vertigo, and neurologic deficits—cerebellar infarct, vertebral artery dissection, and lateral medullary syndrome.
Cerebellar Infarct
Ischemic and hemorrhagic cerebellar infarcts accounts for just 2% of CVAs overall.2 The classic triad of presenting symptoms is headache, vomiting and ataxia.3 Patients typically appear toxic, manifesting dysmetria and ataxia (eg, 71% are unable to walk without assistance).4 A key exam finding is direction-changing nystagmus, in which the direction of saccadic beats follow the direction of gaze. While neurologic deficits that correlate with cerebellar injury are commonly evident, manifestations can be highly variable — making this a remarkably challenging diagnosis. For example, a subset of approximately 10% of patients will present with isolated subjective vertigo without deficits.5 However, among this subset without neurologic deficits, 84% will have either ataxia or direction changing nystagmus. While CT can reliably detect cerebellar hemorrhage,
it has poor sensitivity (26%) for ischemic lesions. Therefore, MRI is the optimal study.5
Vertebral Artery Dissection
Vertebral artery dissection (VAD) is a rare disease entity that typically afflicts relatively young patients. Presenting symptoms are usually acute onset neck pain or headache, followed by progressive development of neurologic symptoms.6 Headaches are typically posterior, ipsilateral, throbbing, and accompanied by diplopia and vomiting. Neurologic deficits often involve ataxia and even hemiparesis. It should be noted that vertebral artery dissection afflicts the posterior circulation and manifests as cerebellar dysfunction—in distinction from carotid dissection, where the presenting headache is associated with contralateral cerebral hemispheric deficits and may be accompanied by Horner's syndrome in up to half of cases. CTA has been shown to be equivalent to MRA and is the initial study of choice to evaluate for VAD in the ED after a hemorrhagic etiology has been ruled out with non-contrast head CT.7 Common neuroimaging findings suggestive of VAD are the “string of pearls” sign, arterial dilation, pseudoaneurysm, double lumen or intimal flap formation (Figure 1).
Lateral Medullary Syndrome
Also known as Wallenberg syndrome, lateral medullary syndrome is a classic constellation of symptoms associated with a medullary infarct involving the posterior inferior cerebellar artery (PICA). Several bordering brainstem nuclei and tracts can be damaged, causing the triad of Horner syndrome, ipsilateral ataxia, and contralateral hypoalgesia. Lateral medullary syndrome is the most common syndrome associated with intracranial vertebral artery occlusion and can be seen in up to 20% of cases of VAD.8,9 Symptoms often include imbalance, vertigo, diplopia, nystagmus, dysphagia, hoarseness, and Horner syndrome. While manifesting symptoms can be variable given degree of infarct, crossed hemisensory deficits are seen in 90% cases.10 An ischemic process within the thrombolysis window warrants immediate evaluation for potential administration of thrombolytics. Emergent labs and neuroimaging should be obtained STAT. While MRI is the gold standard for brainstem strokes, the sensitivity within 48 hours of symptom onset is 72%, further emphasizing importance of clinical suspicion.11
Management
The approach to headache and vertigo should incite prompt evaluation of ABCs, fingerstick glucose, and history and physical, in addition to a thorough neurologic exam with a focus on coordination and ataxia. Any suspicion of stroke should prompt immediate neuroimaging. The HINTS exam may be especially useful in identifying 3 particularly worrisome findings—multidirectional nystagmus, vertical skew, and a normal head impulse test.11 Obtaining a clear history of symptom onset is critical, as thrombolysis may be indicated. Initial imaging of choice is CT/CTA head and neck to evaluate for hemorrhage, dissection and large vessel filling defects. The definitive imaging modality to evaluate brainstem and posterior fossa stroke is MRI/MRA, but this is often not available rapidly enough to influence the decision on thrombolytics. In cases of non-hemorrhagic, ischemic posterior CVAs in which thrombolysis is not indicated, early antiplatelet should be initiated in the ED.
Finally, a broader differential of stroke mimics may prompt lumbar puncture to evaluate for basilar meningitis or multiple sclerosis. The approach to a patient presenting with headache and vertigo must be steeped in a “worst-first” differential diagnosis that evaluates for highly morbid catastrophic cerebellar and brainstem strokes. Prompt evaluation, diagnostic testing, and imaging are crucial to facilitate involvement of consultants and timely, life- and brain-saving interventions.
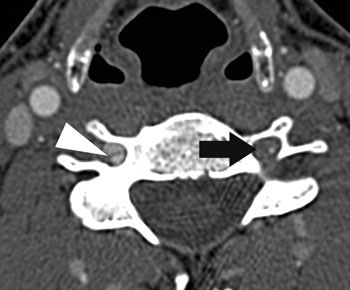 Figure 1. Bilateral Vertebral Artery Dissection on Axial CT. Note intimal flap (arrowhead) and stenotic lesion (arrow). Permission obtained for reproduction; image source: Mathieu H. Rodallec, MD, Véronique Marteau, MD, et al. Craniocervical Arterial Dissection: Spectrum of Imaging Findings and Differential Diagnosis. RadioGraphics 2008;28:1711-1728. Figure 13.
Figure 1. Bilateral Vertebral Artery Dissection on Axial CT. Note intimal flap (arrowhead) and stenotic lesion (arrow). Permission obtained for reproduction; image source: Mathieu H. Rodallec, MD, Véronique Marteau, MD, et al. Craniocervical Arterial Dissection: Spectrum of Imaging Findings and Differential Diagnosis. RadioGraphics 2008;28:1711-1728. Figure 13.Other Considerations
Generally, in the absence of multiple prodromal symptoms, heavy vomiting, ataxia, headache, or neck pain, a peripheral cause is more likely. Three common peripheral etiologies for vertigo are benign paroxysmal positional vertigo (BPPV), vestibular neuritis, and Ménière's disease. Episodic “paroxysms” of vertigo induced by head movements with fatigable nystagmus are typical for BPPV. A positive (abnormal) head impulse test on HINTS would be expected in a post-viral vestibular neuritis, as decreased unilateral vestibular tone alters the vestibulo-ocular reflex. Hearing loss, tinnitus, or aural fullness in context of isolated vertigo is very suggestive of endolymphatic buildup characteristic of Ménière's disease.
TABLE 1. Can't-Miss Dizzy Diagnoses and Preferred Imaging Modality
| Characteristic Features | Imaging | |
| Cerebellar infarct | Headache, vomiting, multi-directional nystagmus, ataxia | MRI/MRA |
| Vertebral artery dissection | Younger patients with neck pain or headache followed by cerebellar symptoms |
CTA = MRA |
| Lateral medullary syndrome | Horner syndrome, “crossed findings” on motor-sensory exam | MRI/MRA |
| BPPV | Paroxysms of vertigo induced by head movements with fatigable nystagmus | N/A |
| Vestibular neuritis | Recent viral illness, abnormal head impulse test on HINTS | N/A |
| Ménière's disease | Hearing loss, tinnitus, or aural fullness with isolated vertigo | N/A |
Case Conclusion
A CTA of the head and neck did not identify any mass, cerebellar hemorrhage, arteriovenous malformation (AVM), or vascular dissection. Lumbar puncture was also normal, including a normal cell count, gram stain, glucose, and absence of oligoclonal bands. An MRI showed no acute intracranial pathology. The patient was admitted to neurology with the presumptive diagnosis of ophthalmoplegic migraine and was treated with dopamine antagonists and NSAIDs. During her 2-day admission, her headache and vertigo resolved. She had regained the majority of the adduction of her eye, and the amplitude of her nystagmus had diminished. Verapamil was initiated as prophylactic migraine treatment and she was discharged with follow-up in neurology clinic.
Learning Points
Acute vestibular syndrome, defined as rapid onset dizziness accompanied by nystagmus, nausea, vomiting, and gait unsteadiness, envelops both benign peripheral and highly morbid central pathologies. However, discerning between these etiologies is particularly challenging when relying on subjective sensory abnormalities amidst a confusing array of descriptions (ie, dizziness, presyncope, vertigo, unsteadiness) that are highly subjective and historian-dependent.14 Further, patients' descriptions of vertigo have been shown to be inconsistent and unreliable.15 The emergency physician must pay close attention to details regarding associated symptoms and always perform a thorough neurological examination.
References
- Counselman FL, Borenstein MA, Chisholm CD, et al. The 2013 Model of the Clinical Practice of Emergency Medicine. Acad Emerg Med. 2014;21(5):574-598.
- Tohgi H, Takahashi S, Chiba K, Hirata Y. Cerebellar infarction. Clinical and neuroimaging analysis in 293 patients. The Tohoku Cerebellar Infarction Study Group. Stroke. 1993;24(11):1697-1701.
- Hotson JR, Baloh RW. Acute vestibular syndrome. N Engl J Med. 1998;339(10):680-685.
- Lee H, Sohn SI, Cho YW, et al. Cerebellar infarction presenting isolated vertigo: frequency and vascular topographical patterns. Neurology. 2006;67(7):1178-1183.
- Chalela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369(9558):293-298.
- Debette S, Grond-ginsbach C, Bodenant M, et al. Differential features of carotid and vertebral artery dissections: the CADISP study. Neurology. 2011;77(12):1174-1181.
- Provenzale JM, Sarikaya B. Comparison of test performance characteristics of MRI, MR angiography, and CT angiography in the diagnosis of carotid and vertebral artery dissection: a review of the medical literature. AJR Am J Roentgenol. 2009;193(4):1167-1174.
- Lee MJ, Park YG, Kim SJ, Lee JJ, Bang OY, Kim JS. Characteristics of stroke mechanisms in patients with medullary infarction. Eur J Neurol. 2012;19(11):1433-1439.
- Kim JS. Pure lateral medullary infarction: clinical-radiological correlation of 130 acute, consecutive patients. Brain. 2003;126(Pt 8):1864-1872.
- Day GS, Swartz RH, Chenkin J, Shamji AI, Frost DW. Lateral medullary syndrome: a diagnostic approach illustrated through case presentation and literature review. CJEM. 2014;16(2):164-170.
- Kattah JC, Talkad AV, Wang DZ, Hsieh YH, Newman-toker DE. HINTS to diagnose stroke in the acute vestibular syndrome: three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke. 2009;40(11):3504-3510.
- Lal V, Sahota P, Singh P, Gupta A, Prabhakar S. Ophthalmoplegia with migraine in adults: is it ophthalmoplegic migraine?. Headache. 2009;49(6):838-850.
- Gelfand AA, Gelfand JM, Prabakhar P, Goadsby PJ. Ophthalmoplegic "migraine" or recurrent ophthalmoplegic cranial neuropathy: new cases and a systematic review. J Child Neurol. 2012;27(6):759-766.
- Newman-toker DE, Edlow JA. TiTrATE: A Novel, Evidence-Based Approach to Diagnosing Acute Dizziness and Vertigo. Neurol Clin. 2015;33(3):577-599, viii.
- Newman-toker DE, Cannon LM, Stofferahn ME, Rothman RE, Hsieh YH, Zee DS. Imprecision in patient reports of dizziness symptom quality: a cross-sectional study conducted in an acute care setting. Mayo Clin Proc. 2007;82(11):1329-1340.