Ultrasound is a powerful tool in the emergency department for the estimation of left ventricular ejection fractions (LVEF).
Although emergency physicians can be accurate at visual estimation of the LVEF,1,2 it can still be useful to know how to obtain quantitative measurements. Objective measurements can be compared to prior values, can be communicated and recorded, and can help new learners inform their ability to visually estimate cardiac function. Much of this is similar to our prior discussion of E-Point Septal Separation (EPSS), but what if the patient is known to have mitral stenosis or the many other reasons that limit your ability to obtain EPSS measurements? It’s good to have more tools. Here we will discuss two other simple measurements that estimate LVEF with good accuracy.
Left ventricular fractional shortening
Left ventricular fractional shortening (LVFS) refers to the fraction the left ventricle shortens during a cardiac cycle. At end diastole the left ventricle will have the largest volume and therefore the “tallest” distance from the far to near wall (septal and free wall respectively) when looking at the left ventricle in the parasternal long axis (PSLA). Said slightly differently, at left ventricle end diastole the left ventricle has its largest diameter (LVEDD). Conversely, at end systole the septal and free walls will be at their closest point in the cardiac cycle (shortest distance) and produce a smaller diameter (LVESD). The fraction of these two distances/diameters can be used as a surrogate for the LVEF itself.
FS%= (LVEDD-LVESD)/LVEDD×100%
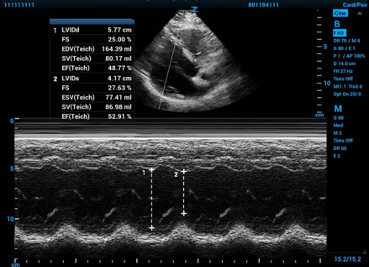
Fractional shortening can be calculated with measurements from standard 2-D echocardiography by measuring the internal diameter of the mid-left ventricular chamber. To get an accurate measurement you must measure perpendicular to the long axis of the left ventricle itself, be careful to not include the papillary muscles or valves, and measure from endocardium to endocardium and not include the ventricular walls. Alternatively, you can place an M-mode spike and use the resulting tracing to measure the difference in the same two diameters. The ultrasound machine will then use equations that luckily you do not have to remember to convert fractional shortening percentage to a LVEF because–and very important to know–the number you get for fractional shortening percent will NOT be the LVEF.
Figure 1. Fractional shortening calculated using M-mode in the parasternal long axis window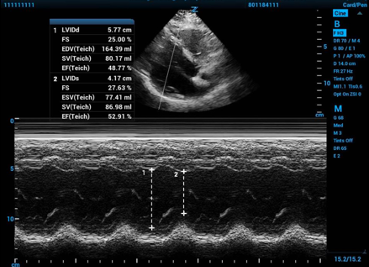
Fractional area change
A similar technique to measure LVEF is fractional area change (FAC). Like LVFS, FAC measures the change in circumferential area of the LV throughout the cardiac cycle–the difference in left ventricular diastolic area (LVEDA) and left ventricular systolic area (LVESA). However, rather than using a M-mode spike, FAC uses planimetry, or tracing of the anechoic blood along the wall of the endocardium. The absolute change in area between diastole and systole directly correlates to LVEF. The measured FAC may then be converted for an approximation for LVEF. Fortunately, modern ultrasound machine algorithms automatically convert FAC to report an LVEF.
FAC = (LVEDA−LVESA)/LVEDA×100%.
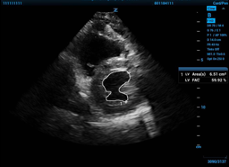
To get accurate FAC measurements, obtain a standard 2-D echo parasternal short axis view at the mid papillary level. Neither myocardium nor papillary muscles should be included, and measurements are taken at the maximum diameter of end diastole, when area is the greatest, and at the smallest diameter at end systole. Again, the ultrasound software will convert the FAC you measure into a LVEF you can use.
Figure 2a and Figure 2b. On the left, the internal area of the LV traced at end diastole, and then on the right, at end systole.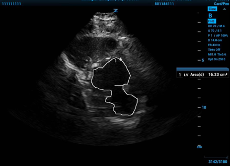
When devised, both FAC and LVFS were compared to an angiographic and a radionuclide based measurement and showed high correlation coefficients indicating they were a good estimation of LVEF.3, 4 Other strengths of these measurements are that they utilize basic echocardiographic windows that are routinely obtained when doing bedside echocardiography and that they are simple and intuitive. Although not completely immune, FAC is less impacted by focal wall motion abnormalities (FWMA) compared to LVFS and remains a reliable measure of LVEF despite mild and moderate FWMAs.4
The gold standard 2-D echocardiogram measurement of LVEF is currently the Simpson biplane method,5 which is essentially a much more rigorous LVFS/FAC technique that looks at the left ventricle in two planes instead of the one in LVFS and at 20 points in the left ventricle instead of the one point in both LVFS and FAC. All three measurements/methods work because both blood and the myocardium are practically incompressible, and so changes in left ventricle volume (or diameter alone in LVFS’ case) correlate with ejection fraction.
The differences in the Simpson biplane method and LVFS/FAC highlight LVFS and FAC’s shortfalls though. Both of these techniques can be falsely low when the measured area involves a wall motion abnormality or falsely high if one is present and not included in the measured area. Additionally, both of these modalities are highly preload and afterload dependent.4 Inaccuracies in measurements–be it from: misplaced calipers, non-perpendicular measurements, incorrectly included valvular areas or papillary muscles, or wall motion abnormalities–will not be averaged out for LFVS or FAS given they are singular point measurement. LVFS has also been shown to underestimate LVEF in patients with concentric left ventricular hypertrophy.6
LFVS and FAC are quick measurements to obtain from windows we are already obtaining in point-of-care echocardiography. They allow for new learners to build a gestalt they can use later when visually estimating LVEF, and they allow for us to communicate our findings with our colleagues. Next time you are doing an echo in the emergency department try these measurements and build up your critical care ultrasound skill-set and prepare for more advanced techniques to come.
References
- Ünlüer Erden E, Karagoz A, Akoglu H, Bayata S. Visual estimation of bedside echocardiographic ejection fraction by emergency physicians. West J Emerg Med.2014;15(2):221.
- Moore CL, Rose GA, Tayal VS, Sullivan DM, Arrowood JA, Kline JA. Determination of left ventricular function by emergency physician echocardiography of hypotensive patients. Acad Emerg Med. 2002;9(3):186-193.
- Quinones MA, Waggoner AD, Reduto LA, et al. A new, simplified and accurate method for determining ejection fraction with two-dimensional echocardiography. Circulation. 1981;64(4):744-53.
- Suresh Chengode. Left ventricular global systolic function assessment by echocardiography. Ann Cardiac Anesth. 2016; 19(Suppl 1): S26–S34.
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1-39.e14.
- Maciver DH. The relative impact of circumferential and longitudinal shortening on left ventricular ejection fraction and stroke volume. Exp Clin Cardiol. 2012;17(1):5-11.