This case shows a rare presentation of newly diagnosed HOCM in an elderly patient who came to the emergency department after an episode of syncope.
The Case
A 94-year-old female was brought to the ED by EMS reporting syncope and dyspnea. EMS reported finding the patient to be hypoxic and hypotensive. They provided supplemental oxygen via nasal cannula, started a dopamine infusion, and transported her to the ED, where a non-rebreather mask was placed secondary to persistent hypoxia. She remained hypotensive and was subsequently started on vasopressor support with vasopressin and norepinephrine infusions.
Initial EKG did not show any signs of arrhythmias. Initial diagnostic labs were concerning for mildly elevated creatinine compared to the patient’s baseline and an elevated BNP. Chest X-ray revealed mild vascular congestion but no signs of acute cardiopulmonary abnormalities. Due to the concern for pulmonary embolism, a CT angiogram of the chest was obtained, which was unremarkable. She was then admitted to the critical care teaching service secondary to requiring vasopressors. An echocardiography performed during admission showed mild left ventricular outflow obstruction is present with findings consistent with hypertrophic obstructive cardiomyopathy. She also developed new onset atrial fibrillation during her hospital course.
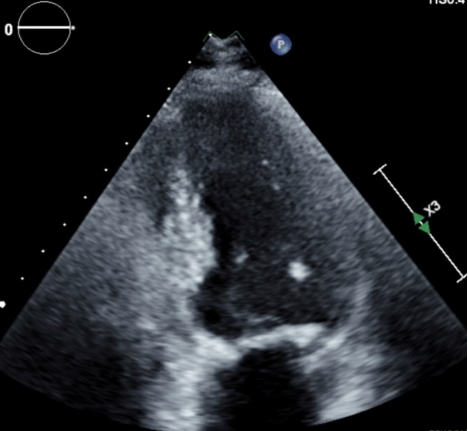
Figure 1. LVOT obstruction during systole
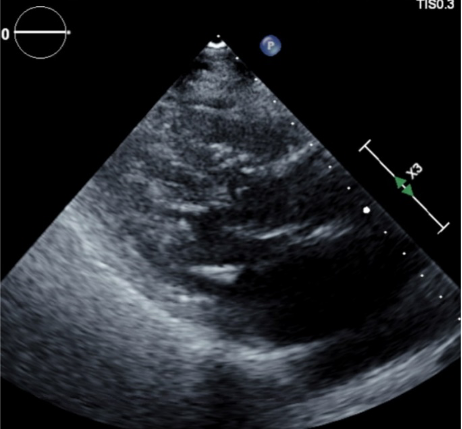
Figure 2. Hypertrophy of the interventricular septum with obstruction during systole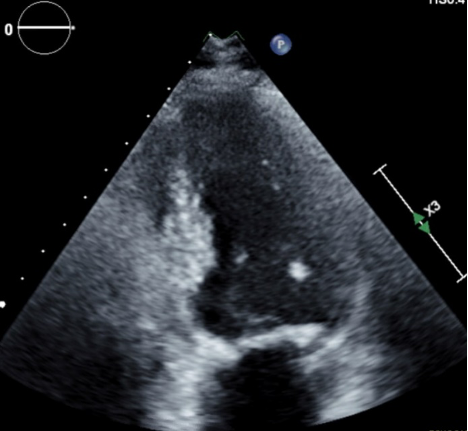
Discussion
Hypertrophic cardiomyopathy (HCM) is a well-known and researched genetic disease that leads to hypertrophy of the myocardium, generally most prevalent within the ventricular walls. The thickening of the heart muscle leads to left ventricular stiffness, mitral valve changes, and cellular changes to the myocardium. Hypertrophic obstructive cardiomyopathy (HOCM) is a subset of HCM, in which the hypertrophy of the ventricular walls results in obstruction of the ventricular outflow tract. It is the leading cause of non-traumatic sudden death in young individuals and most well-known for causing sudden cardiac death in athletes. While most known for being present in young individuals, it is diagnosed in patients of all ages. It has a prevalence of approximately 1:500.1 HOCM is caused by mutations of dozens of genes encoding sarcomere-associated proteins. The genes most found to be mutated are MYH7 and MYBPC3, which encode beta-myosin heavy chain and myosin-binding protein C, respectively. They account for ~50% of HOCM families.2 Inheritance is primarily autosomal dominant with variable penetrance. HCM is a relatively benign disease, with approximately two thirds of patients living a normal life span without significant morbidity. Those that suffer adverse effects generally result due to severe left ventricular outflow tract obstruction leading to increased risk of sudden cardiac death (SCD) and heart failure. Major risk factors that increase the probability for SCD occurring includes family history of SCD, recurrent syncope caused by arrythmias, sustained and repetitive non-sustained ventricular tachycardia, and a previous episode of cardiac arrest. Studies have found that individuals who are diagnosed at advanced age (>60 years) are generally at a decreased risk for SCD and consider it a negative risk marker. This is likely secondary to myocardial stabilization despite the genetic mutation, as well as the ability for the heart to tolerate chronic LV outflow obstruction appropriately.3
Presentation
Patients with HOCM will generally present to the ED as a result of chest pain, presyncope, syncope, or palpitations. Chest pain results secondary to myocardial hypoperfusion due to the hypertrophic ventricular wall, which in turn leads to a myocardial oxygen supply and demand imbalance. This results in ischemic chest pain that may present similarly to anginal pain with crushing chest pain radiating to the left arm and neck. Patients that develop arrythmias secondary to HOCM will present with syncope, presyncope, or palpitations. The most common arrythmia produced is recurrent non-sustained ventricular tachycardia. As with the patient presented in this case, atrial fibrillation occurs in approximately 25% of patients with HOCM and LVOT obstruction. It is unclear what the mechanism for development of atrial fibrillation is at this time.4 Syncope can also result secondary to left ventricular outflow tract obstruction in the setting of preload reduction as well as a sudden increase in myocardial stress. Common causes of preload reduction include hypovolemia, hemorrhage, and medications effects. Myocardial stress can occur in the setting of exertion such as exercise or playing sports, as well as in the setting of emotional stress.
Diagnosis
Initial evaluation in the Emergency department should begin with an EKG to evaluate for cardiac arrythmias as well as for signs of left ventricular hypertrophy. EKG findings concerning for HOCM are variable in nature. One group of findings are increased precordial voltages with non-specific ST segment and T wave abnormalities with the voltages meeting voltage criteria for LVH. Asymmetrical septal hypertrophy results in deep, narrow (“dagger-like”) Q waves in the lateral (V5-6, aVL) and inferior (II, III, aVF) leads as seen in the EKG below.
Figure 3. EKG findings of HCM with dagger Q waves in the lateral and inferior leads.5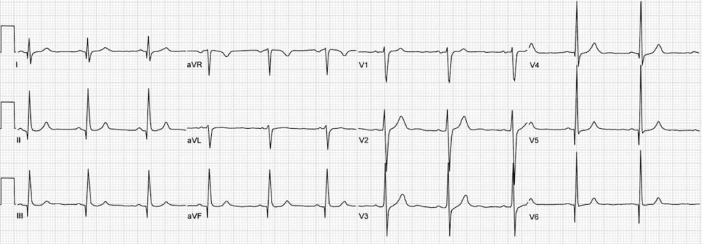
A second and less prevalent form of HCM is apical HCM. This variant leads to localized hypertrophy to the left ventricular apex leading to a “spade-shaped” configuration of the LV on echocardiography. EKG findings of this include giant T-wave inversions in the precordial leads, as seen in the EKG below.
Figure 4. EKG findings of apical HCM with giant T-wave inversions in the precordial leads.5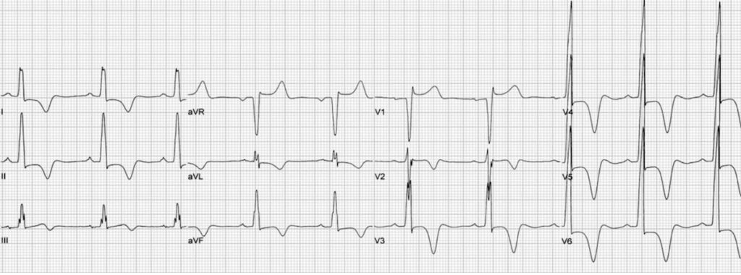
The diagnosis of HOCM is commonly confirmed with an echocardiogram and is generally not completed until a patient is admitted into the hospital. The presence of left ventricular end diastolic wall thickness >13mm is needed to make the diagnosis.
Figure 5. Echocardiographic images of HOCM6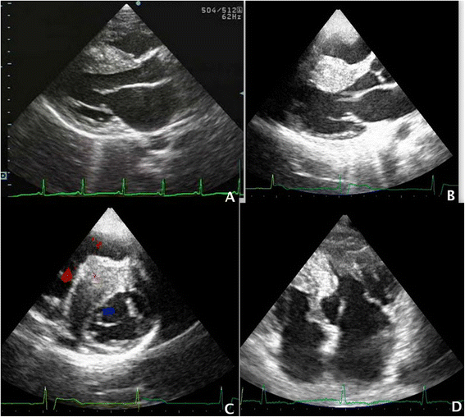
Treatment/Disposition
Many individuals that are diagnosed with HCM have a relatively benign course and do not require any significant interventions. Those who present to the ED should, however, pique a higher clinical suspicion for requiring interventions as they are symptomatic from the condition. For individuals presenting with anginal chest pain, beta-adrenergic receptor blockers have been found to relieve chest discomfort by decreasing myocardial oxygen demand. They have also been shown to attenuate exercise induced LVOT obstruction that results in dyspnea. Non-dihydropyridine calcium channel blockers such as verapamil or diltiazem have also been found to have benefit when patients do not tolerate or respond to beta-blockers. Individuals who present with new onset arrythmias such as atrial fibrillation are most responsive to cardioversion. If patients develop persistent or paroxysmal atrial fibrillation, they will require long-term anticoagulation to reduce the risk of thromboembolism. In individuals with increased risk factors for SCD, implantation of an implantable cardioverter defibrillator (ICD) is strongly advised. Major risk factors for SCD include SCD include family history of SCD, recurrent syncope caused by arrythmias, sustained and repetitive non-sustained ventricular tachycardia, and previous episode of cardiac arrest. Individuals with any of these risk factors are likely to benefit the most from having an ICD implanted. For individuals who develop severe heart failure that is not improved with pharmacotherapy, they may eventually require implantation of a left ventricular assistance device (LVAD) or cardiac transplantation.
Conclusion
This case presentation demonstrated a rare presentation of newly diagnosed HOCM in an elderly patient that presented secondary to syncope. She developed concern for cardiogenic vs. hypovolemic shock, requiring multiple vasopressors for cardiovascular support. She also developed new onset atrial fibrillation to further complicate the presentation and hospital course. Given the multiple possible causes for her syncope and persistent hypotension, the inciting event in this case remains unclear. The case teaches the importance of keeping a wide differential.
The patient was resuscitated in the ED, diagnosed on hospital day 1 (aiding in timely treatment) and was discharged home with a prescription for metoprolol after a brief hospital stay.
References
- Maron, B. J., Ommen, S. R., Semsarian, C., Spirito, P., Olivotto, I., & Maron, M. S. (2014). Hypertrophic cardiomyopathy: present and future, with translation into contemporary cardiovascular medicine. Journal of the American College of Cardiology, 64(1), 83–99. https://doi.org/10.1016/j.jacc.2014.05.003
- Marian, A. J., & Braunwald, E. (2017). Hypertrophic Cardiomyopathy: Genetics, Pathogenesis, Clinical Manifestations, Diagnosis, and Therapy. Circulation research, 121(7), 749–770. https://doi.org/10.1161/CIRCRESAHA.117.311059
- Hypertrophic Cardiomyopathy in Patients of Advanced Age. (2015, August 17). American College of Cardiology. https://www.acc.org/latest-in-cardiology/articles/2015/08/17/13/12/hypertrophic-cardiomyopathy-in-patients-of-advanced-age
- Olivotto, I., Cecchi, F., Casey, S. A., Dolara, A., Traverse, J. H., & Maron, B. J. (2001). Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation, 104(21), 2517–2524. https://doi.org/10.1161/hc4601.097997
- Burns, E. (2019, March 16). Hypertrophic cardiomyopathy. Life In The Fastlane. https://litfl.com/hypertrophic-cardiomyopathy-hcm-ecg-library/
- Parato, V.M., Antoncecchi, V., Sozzi, F. et al. Echocardiographic diagnosis of the different phenotypes of hypertrophic cardiomyopathy. Cardiovasc Ultrasound 14, 30 (2015). https://doi.org/10.1186/s12947-016-0072-5