A 63-year-old female with past medical history of COPD (on 2L home oxygen), hypertension, “some remote heart surgery,” presents to the emergency department by EMS with acute, increasing shortness of breath over the past 12 hours. Patient denies fever, increased cough or recent travel.
Initial vital signs were HR 118, RR 35, SpO2 61% on 2L and BP 124/86.
On physical exam the patient is alert and in severe respiratory distress, with diminished but clear breath sounds bilaterally.
The patient’s respiratory status fails to improve with non-rebreather mask or Bi-level Positive Airway Pressure and ultimately is intubated.
Laboratory work reveals no leukocytosis, no lactic acidosis, normal hemoglobin and VBG with pH 7.28 and CO2 >115. Chest-x-ray shows cardiomegaly with prominent pulmonary vasculature. Computed tomography pulmonary angiography reveals no pulmonary embolism, but marked dilation of the pulmonary trunk.
The patient required medical intensive care unit admission and was discharged 3 weeks later on her baseline oxygen requirement.
DISCUSSION
Introduction: Pulmonary hypertension can be a complicating factor in many patient presentations, especially in the acute setting. It is defined as a resting mean pulmonary artery pressure (PAP) at or above 25mm Hg, and is typically diagnosed via right heart cardiac catheterization.1 There are currently five categories of pulmonary hypertension (PH). These include: pulmonary arterial hypertension (PAH - group 1), PH due to left sided heart disease (group 2), PH secondary to chronic hypoxic lung disease (group 3), PH secondary to chronic thromboembolism (group 4) and PH secondary to unclear/multifactorial pathogenesis (group 5).2 Pulmonary arterial hypertension (group 1) is often idiopathic or occurs as a result of a connective tissue disorders. Of the groups above, pulmonary hypertension secondary to left sided heart disease (group 2) is the most common. Our patient would fall into group 3 due to her history of COPD, and PH is important to keep in mind when evaluating patients presenting with presumed COPD exacerbations. It is vital to be able to identify and begin appropriate treatment for PH sooner rather than later, as it vastly improves patient outcomes and their quality of life.
Pathophysiology: The exact pathophysiology varies based on the underlying mechanism. In the case of PAH, it is characterized by vascular remodeling and accompanied by fibrosis, inflammation, and abnormal proliferation of endothelial and vascular smooth muscle cells.3 The mechanism is thought to be secondary to endothelial dysfunction with an imbalance between endogenous vasodilators (eg, prostacyclin) and vasoconstrictors (endothelin-1) resulting in a net effect of vasoconstriction and thrombus formation. There are three major pathways (nitric oxide, endothelin and prostacyclin) playing a role in the development and progression of PAH.4 Pulmonary vascular smooth muscle cells that normally have a low rate of multiplication undergo proliferation and hypertrophy leading to intimal narrowing and increased resistance to blood flow. Furthermore, circulating platelets in patients with PAH are in a continuous state of activation and contribute to the prothrombotic state by aggregating at the level of the injured endothelial cells.
In group 2 PH, left-sided heart disease leads to pulmonary venous congestion; eventually producing increased pulmonary arterial pressure. Group 3 results from chronic hypoxemia-induced pulmonary vasoconstriction and thus hypertension.5 Group 4 involves chronic thromboembolism, which impedes normal blood flow within pulmonary vessels. This results in vascular congestion, pulmonary hypertension, and right ventricle strain. Group 5 is by definition unclear, and often has no clear pathophysiology.4
Signs and Symptoms: Approximately 86% of patients with PH will present with shortness of breath. As a nonspecific symptom, PH is often overlooked.6 Additional associated symptoms can include fatigue, chest pain, exertional syncope, light-headedness, edema and palpitations. Initially, a loud S2 may be the only discernable sign on examination. However, as PH progresses, signs may include a pansystolic murmur of tricuspid regurgitation, diastolic murmur of pulmonary insufficiency, left parasternal lift, jugular venous distension, hepatomegaly, peripheral edema and ascites.2 These signify right ventricular failure due to increased pressure in the pulmonary system.
Diagnosis: Definitive diagnosis is established via right heart catheterization.3 However, in the emergency department we rely on more accessible modalities to indicate the presence of PH such as EKG, labs, and imaging. Evidence of right heart strain on EKG, such as a right bundle branch block or t wave inversions and ST depression in the anterior leads are associated with PH.7 The most common electrocardiogram finding is right axis deviation (Figure 1). Right ventricular hypertrophy may also be noted, with V1 and V2 having large R waves and smaller S waves. V5, V6, I and aVL may also display smaller R waves than normal. Prolongation of the QTc interval or QRS complex likely indicate more advanced disease.7
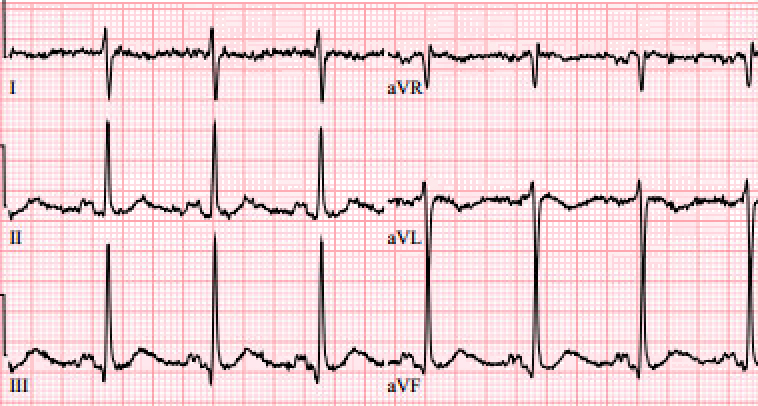
Figure 1.
Although not emergently indicated, a troponin level may be useful, especially if there is any concern for ischemia due to poor right coronary artery perfusion. An elevated troponin in the setting of pulmonary hypertension is associated with increased morbidity and mortality. A brain-natriuretic peptide level may also prove useful if it is elevated from the patient’s baseline, as it reflects increased myocardial stretch.2 Chest x-rays (CXR) can offer further evidence of pulmonary hypertension, as they may show cardiomegaly or prominent central pulmonary arteries (Figure 2). Specifically, right ventricle and atrium enlargement can be seen in advanced cases. Chest x-ray can also offer evidence of underlying disease processes leading to pulmonary hypertension; for example, hyperinflation as seen in COPD or interstitial lung fibrosis.8
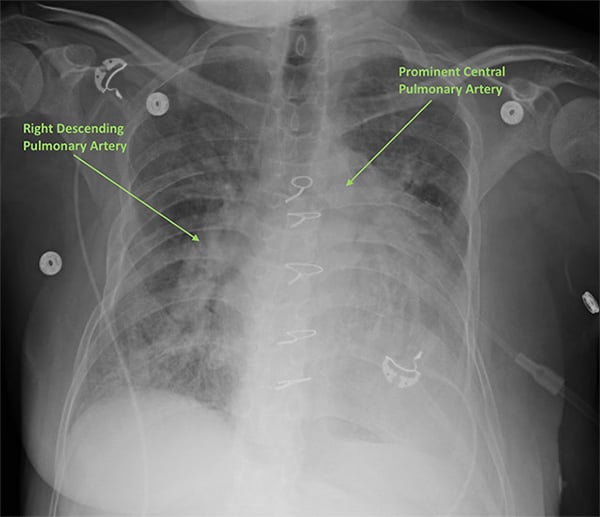
Figure 2.
A computed tomography (CT) scan of the chest can also be useful in diagnosis if the main pulmonary artery diameter is >29mm and/or the ratio of the main pulmonary artery to the ascending aorta diameter is >1.7 CT will reveal enlargement of the right ventricle, mediastinal abnormalities, and other findings that will help clarify the diagnosis (Figure 3).
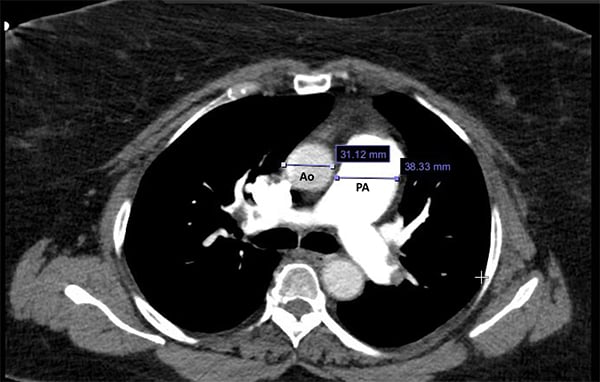
Figure 3.
Bedside ultrasound and echocardiogram, can be performed quickly and provide key information to expedite the diagnosis. An RV: LV ratio >1 on the apical four-chamber view indicates right ventricular overload (Figure 4). The “D” sign may be visualized in the parasternal short-axis view (Figure 5). This is produced when the right ventricle is enlarged, leading to interventricular septum flattening during diastole, which in turn results in a D-shaped left ventricle.9 Ventricular wall thickening will differentiate chronic vs. acute right heart. In acute strain, as seen with a pulmonary embolism, we would see a thin, free wall; however, the chronic strain characteristic of PH would produce a thickened wall. Like CT, ECHO can also be useful in determining whether there is another disease process playing a role in the patient’s presentation.
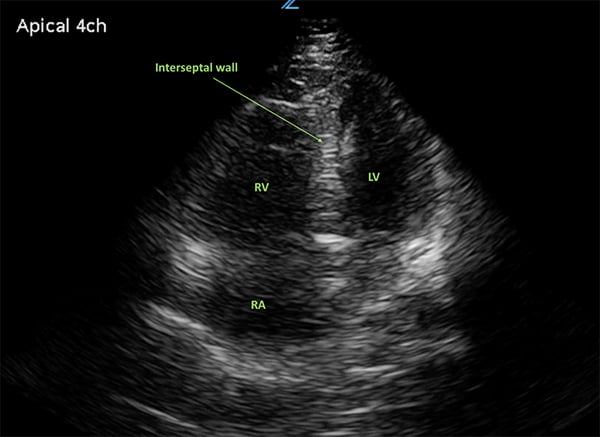
Figure 4.
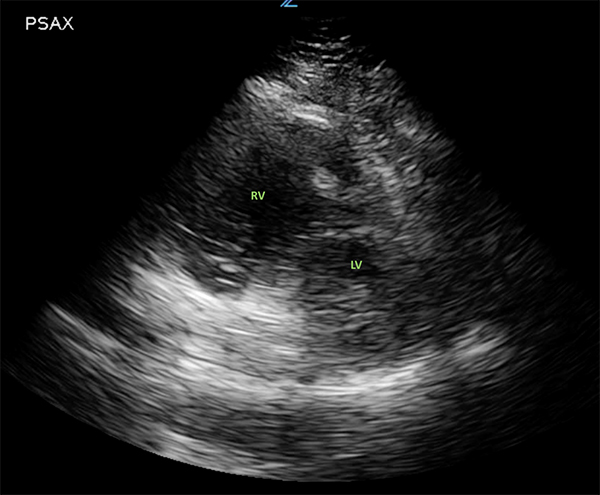
Figure 5.
Management: Outpatient management may include a combination of calcium channel blockers, digoxin, diuretics, home oxygen, anticoagulation, endothelin receptor antagonists, phosphodiesterase inhibitors and prostacyclin analogues. However, our chief concern is acute management.
1. Airway management can prove challenging because positive pressure ventilation (PPV) and intubation increase the risk of cardiovascular collapse. The increased intrathoracic pressure from positive pressure ventilation puts additional strain on the heart by decreasing preload, which worsens cardiac output.3 If intubation cannot be avoided, Etomidate should be used for induction due to minimal effects on systemic vascular resistance, pulmonary vascular resistance and cardiac contractility. Lung protective settings should be embraced with a tidal volume of 6mL/kg ideal body weight and the lowest PEEP to maintain oxygen saturation >90%.10 Monitor serial plateau pressures, as these should be kept low.3
2. Oxygenation: hypoxemia and hypercapnea cause vasoconstriction of the lungs and worsening pulmonary vascular resistance. A goal of SaO2>90% is preferable, as these patients cannot tolerate permissive hypercarbia and hypoxemia.3
3. Circulation: fluids must be used judiciously. The right ventricle is already under a great deal of strain and additional fluids can stretch the myocardial fibers and cause decreased cardiac output due to right ventricle failure (Figure 6). Additionally, in chronic PH, right ventricle remodeling leads to elevated transmural pressures, impairing RCA perfusion, which may lead to right ventricular ischemia. Intravenous fluids should only be used if the patient is obviously dehydrated, and even then should be used in small boluses with frequent re-evaluation.3 Hemodynamic stability in these patients is precarious, and can easily be disrupted.
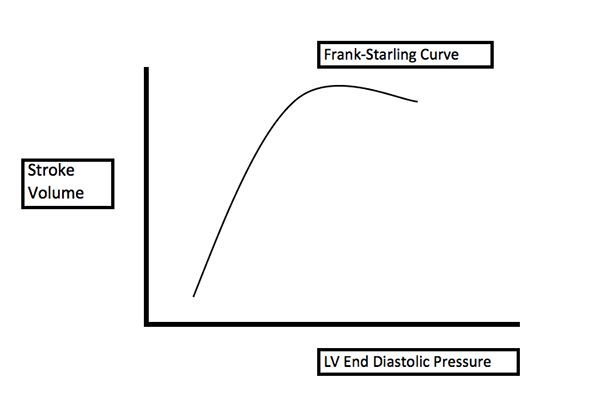
Figure 6.
4. Right ventricle support is critical. The main agents include dobutamine and milrinone. Dobutamine operates via beta-2 mediated systemic vasodilation, causing decreased pulmonary and systemic vascular resistance and increased contractility. Milrinone works as a PDE-3 inhibitor, and reduces peripheral vascular resistance to augment right ventricle function.11 However, it is important to note that these agents may cause hypotension, and vasopressors may be necessary to offset this effect.
5. Vasopressors: norepinephrine is the vasopressor of choice, and reduces the 28-day mortality from cardiogenic shock.12 Other agents that may be considered include epinephrine and vasopressin. Phenylephrine should be avoided since it causes direct pulmonary vasoconstriction, thereby increasing the work of the right ventricle. Dopamine is also not ideal due to increased risk of tachydysrhythmias and elevation in peripheral vascular resistance and pulmonary artery pressure.3
6. Next steps: consider prostanoids, endothelin receptor antagonists, and PDE-5 inhibitors. These agents have half-life of 30 minutes to one hour, and patients may develop severe rebound pulmonary hypertension if their infusion is stopped. Consider starting these agents with expert consultation. Nitric oxide (NO) should be utilized as an inhaled medication when available. Patients with PAH typically have low levels of NO, and the severity of disease inversely correlates with NO reaction products. Nitric oxide causes systemic vasodilation and helps to reduce the stress placed on the right ventricle by decreasing preload.3
7. If all else fails, consider right ventricular assist devices and extracorporeal membrane oxygenation.
8. Disposition: most patients will require inpatient admission, if for no other reason than to identify the underlying mechanism of their pulmonary hypertension. Additionally, intensive care unit admission should be considered for patients with right ventricular failure at the time of presentation.3
Pearls and Pitfalls
- Consider Pulmonary Hypertension when evaluating ED patients with shortness of breath because this condition is under-diagnosed and can be easily missed.
- Give fluids judiciously, carefully manage intravascular fluid load, and avoid positive-pressure ventilation.
- Avoid hypoxia and use vasopressors early to protect the RCA.
- Augment right ventricle function with dobutamine and pulmonary vasodilators to overcome pulmonary vascular resistance.
References
- Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D42-D50.
- Frost A, Badesch D, Gibbs JSR, et al. Diagnosis of pulmonary hypertension. Eur Respir J. 2019;53(1): pii: 1801904.
- Wilcox SR, Kabrhel C, Channick RN. Pulmonary Hypertension and Right Ventricular Failure in Emergency Medicine. Ann Emerg Med. 2015;66(6):619-628.
- Silva S. Pulmonary hypertension pathophysiology. Pulmonary Hypertension News. Available at: https://pulmonaryhypertensionnews.com/pulmonary-hypertension-pathophysiology/ Accessed September 3, 2019.
- Nauser TD, Stites SW. Diagnosis and treatment of pulmonary hypertension. Am Fam Physician. 2001;63(9): 1789-1798.
- Brown LM, Chen H, Halpern S, et al. Delay in Recognition of Pulmonary Arterial Hypertension: Factors Identified from the REVEAL Registry. Chest. 2011;140 (1): 19–26.
- Galiè Nazzareno, Humbert M, Vachiery JL, et al. 2015. 2015 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension. Eur Respir J. 2015;46(4):903-975.
- Chest X-Ray. PAH TV. Available at: http://pah.tv/ResourceCenter/TestingDiagnostics/ChestXRay.aspx. Accessed September 3, 2019.
- Pulmonary Hypertension and the D-Sign. Loma Linda Ultrasound. Available at: https://lluultrasound.org/home/blog/2017/10/23/pulmonary-hypertension-d-sign/. Accessed September 3, 2019.
- Misch M. CritCases 7 Pulmonary Hypertension - a Fine Balance. Emergency Medicine Cases. March 28, 2017. Available at: https://emergencymedicinecases.com/pulmonary-hypertension.
- Eichhorn EJ, Konstam MA, Weiland DS, et al. Differential effects of milrinone and dobutamine on right ventricular preload, afterload and systolic performance in congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1987;60(16): 1329-1333.


