Algal toxins are responsible for between 50,000 and 500,000 human intoxications per year.1 Globally, there are 2,000 cases of human paralytic shellfish poisoning (PSP) reported per year, and acute intoxication demonstrates from 1.5% to 15% mortality rate in the literature.1,2
Paralytic shellfish toxins are found mostly in bivalve shellfish mussels, clams, oysters, scallops, and snails. Monitoring programs in many countries minimize health risks and have reduced human illnesses and fatalities.5,15 Paralytic shellfish toxin is 1,000 times more potent than cyanide and was used in some World War II suicide pills for pilots in case they were captured in enemy territory.
We present a case of the first PSP fatality in Alaska since 2010.
Case Description
A 64-year-old female presented to a clinic in July 2020 on Unalaska Island in the Aleutian Islands. The patient complained of vomiting, generalized weakness, “floating,” and paresthesias around the lips and face. She had a medical history of hypertension and diabetes. Her list of medications included atorvastatin 20 mg by mouth nightly, flonase nasal spray 1 spray each nares daily, glipizide 5 mg by mouth twice daily, lidocaine 5% patches as needed for pain 12 hours on and 12 hours off, losartan 50 mg by mouth once daily, and metformin 1,000 mg twice daily.
The patient was retired and living with family. She was a never-smoker with no reported alcohol or drug intake. Family members initially thought the patient was having a stroke and called for EMS. The patient was transported to the island clinic via ambulance. A thorough history taken by the lead paramedic noted consumption of mussels and snails at 6 pm that were self-harvested earlier in the day. Samples were taken from the house, brought to the clinic, and placed on ice. The patient estimated that she had eaten 12 mussels and 8 snails. Family noted that she ate more than anyone else; a few family members did not consume any shellfish or snails, and the rest of the family denied symptoms and declined transport.
She presented with Glasgow Coma Scale 15 on arrival to the clinic. She was alert and oriented to her date of birth andlocation and recognized her son-in-law, but she could not state the year. She was complaining of weakness, floating, perioral numbness, and paresthesias. Intake vital signs were pulse of 95, blood pressure 168/94, temperature 37.2 C, respirations 16/minute, SPO2 98%, and BMI 31.8. Her initial neurologic exam included intact cranial nerves, strength 5/5 in all four extremities, and a negative Babinski sign. She required coaching throughout the physical exam and had a dissociated-appearing, slow-motion motor exam. With significant coaching, she was able to demonstrate normal range of motion and 5/5 strength in the extremities but was disoriented and distracted during the exam. She had 2+ patella deep-tendon reflexes and undetectable Achilles tendon reflexes. Sensation was intact to touch bilaterally to the face, upper, and lower extremities. She had palpable radial and dorsalis pedis pulses.
Initial CBC, CMP, troponin, coagulation profile, and urinalysis were within normal limits (Figure 1). A 10-panel drug screen was negative. The EKG in the clinic demonstrated a heart rate of 106 bpm, PR interval 192 ms, QRS 90 ms, QT/QTc interval 344/406 ms, P/QRS/T axis of 37/32/26, RV5/SV1 amp 0.695/0.940 mV, and RV5/SV1 amp 1.635 mV, and was read as sinus tachycardia (Figure 2).

Figure 1
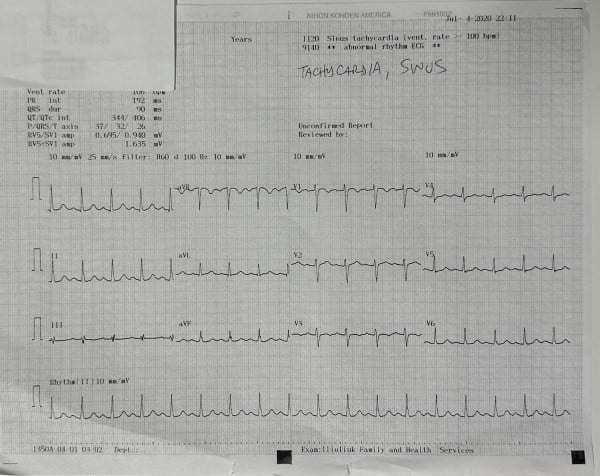
Figure 2
Due to the history of recent self-harvested shellfish consumption, nonfocal neurologic exam, and lack of obvious metabolic or electro-cardio disturbance, paralytic shellfish poison intoxication was suspected. The differential included paralytic shellfish intoxication, medication overdose, cerebellar stroke, or infection.
Historically, patients with paralytic shellfish poison intoxication may require ventilator support. Intubation was the recommendation of Alaska Poison Control if the patient was in respiratory distress or demonstrated hemodynamic compromise and sodium bicarbonate if the QRS on EKG was >100 ms.
The decision was made to transport the patient 3 hours via air medevac to tertiary care, understanding that the island clinic was not capable of providing the level of life support we were expecting she might require. She was protecting her airway and remained hemodynamically stable so intubation was delayed. On EKG the QRS was 90 ms (<100 ms) so intravenous sodium bicarbonate was held.
Two large-bore intravenous access points were established, and intravenous normal saline was started at a rate of 150 ml/hr in the rural clinic on the island. The patient was given oxygen 2 L via nasal cannula, diphenhydramine 25 mg intravenous, methylprednisolone 62.5 mg intravenous, and metoprolol 10 mg intravenous to treat hypertension. The medevac crew was prepared to intubate in flight if needed. The mussels and snails as well as an initial urine sample were placed in a cooler and sent with the patient for the Alaska State Epidemiology Lab to collect at the tertiary care facility in Anchorage. The commercial medevac company transported the patient on continuous cardiac monitor for the 3-hour flight from Unalaska Island to tertiary care in Anchorage. The medevac team was well-prepared, expecting to intubate and support respirations if needed.
The patient was initially stable during the flight. However, two-thirds of the way to Anchorage, she abruptly stopped speaking to the flight crew and went into simultaneous respiratory and cardiac arrest with asystole. There was no precipitating respiratory distress, hypoxia, hypotension, or rhythm, nor change in any vital signs, before the arrest. Advanced Cardiac Life Support protocol was initiated in the medevac plane. She was resuscitated 3 times enroute to Anchorage by the flight crew with return of spontaneous circulation after cardiopulmonary resuscitation the first round and two total doses of epinephrine on subsequent rounds of cardiopulmonary resuscitation.
The patient was brought to the emergency department in Anchorage resuscitated but subsequently went back into asystole alternating with atrial flutter and was resuscitated a reported 8 more times in the ED (11 total). She was on norepinephrine, epinephrine, and sodium bicarbonate infusions in the ED and received calcium, magnesium, and vasopressin additionally. Return of spontaneous circulation was obtained after seconds of cardiopulmonary resuscitation during several episodes of arrest before epinephrine could be administered. The patient was pronounced deceased in the ED. Final comprehensive metabolic panel demonstrated elevated liver function (ALT 781, AST 712) consistent with shock/coded liver and calcium of 12.4 up from 9.7. Sodium was 145 up from 136. EKG in Anchorage demonstrated atrial flutter with rapid ventricular response, left bundle branch block, prolonged QTC, with a ventricular rate of 147 bpm, QT interval of 396 ms, corrected QT interval of 619 ms, QRS axis of 78 degrees, and T axis of 240 degrees.
Family members were repeatedly offered in-clinic monitoring or transport off the island for evaluation but declined and agreed to be monitored for symptoms remotely for 48 hours. They self-reported eating fewer mussels and snails than the patient had consumed (some ate zero) and denied symptoms.
State epidemiology lab personnel picked up the samples for analysis, and the state’s medical examiner took control of the patient for autopsy. The cause of death was determined to be from respiratory and cardiac arrest secondary to paralytic shellfish poison intoxication. The Alaska State Epidemiology Laboratory determined that the samples contained 12,000 mcg/kg. With such a high toxin level in the tested mussels, it was very surprising that family members who had consumed the shellfish did not also become ill.
Discussion
Paralytic shellfish poisoning is caused by eating filter feeders contaminated by paralytic shellfish toxin from algae.1-6The toxin cannot be destroyed by cooking or freezing.2 Paralytic shellfish toxin causes reversible binding of sodium and calcium channels with prolongation of the gating of potassium channels; this causes respiratory paralysis and can stop the heart.2 Toxin levels contained in a single shellfish can be fatal to humans, and shellfish in the same area can contain widely variable levels of toxin.2
Favorable coastal conditions cause increased harmful algae blooms, which lead to more species containing high marine biotoxin burden.16 Extreme weather events including warmer ocean temperatures in the region have been associated with increased harmful algae blooms and increased contaminated seafood.16 In 2019, Barbosa et al demonstrated that, given static harmful algae bloom exposure, certain species of fish had higher biotoxin burden if the temperature of the water was higher.16 In 2019 and 2020, the Southern Bering Sea surface water temperatures reached record highs.17 The Qawalangin Tribe of Unalaska historically collects data on water temperatures and samples mussels in The Aleutian Islands (Figure 3).
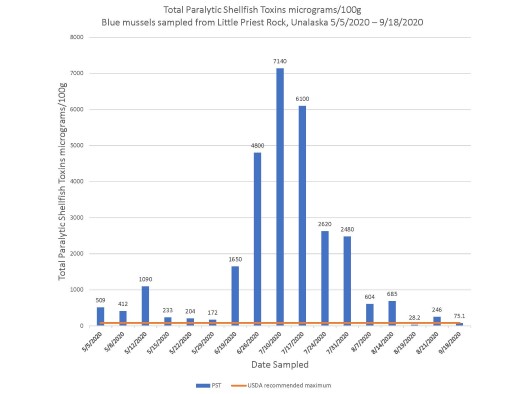
Figure 3
The levels found in the mussels obtained from the patient analyzed by the State of Alaska Environmental Lab were 12,000 mcg/kg. Twelve thousand mcg/kg is 150 times the safe consumption limit of 80 mcg/kg. The death was confirmed in the autopsy report by the Alaska State Medical Examiner to be from “paralytic shellfish poisoning after consuming mussels and snails contaminated with paralytic shellfish toxin self-harvested from Unalaska Island, Alaska”. This was the first paralytic shellfish poisoning fatality reported in Alaska since 2010.
Paralytic shellfish toxin has no known rapid analysis, abnormal laboratory values, or typical vital signs findings. EKG changes, including QRS >100 ms, are late findings. The history and subjective clinical presentation should be thorough and raise suspicion for paralytic shellfish poisoning. Patients should be sent with samples of the suspected paralytic shellfish toxin-containing products consumed, if available, as well as initial urine samples. State epidemiology lab personnel should be notified.
Symptoms of paralytic shellfish poison intoxication can present within minutes to hours. Symptoms include tingling of the lips and tongue, tingling of fingers and toes, loss of muscle control in the arms and legs, difficulty breathing, sense of floating, nausea and vomiting, and autonomic instability. Symptoms can mimic a stroke, so a thorough history is tantamount. The muscles of the chest and abdomen can become paralyzed anywhere from minutes to hours from exposure. With high toxin exposures, death can occur in as little as 2 hours via cardiorespiratory failure.2
In an Alaska case report review, patients who have access to respiratory support can survive paralytic shellfish intoxication. This case is unusual because we were not expecting a simultaneous respiratory and cardiac arrest to occur 8.5 hours after ingestion with her mental status, monitor, and vitals being stable until the arrest. Pre-transport intubation should be considered. As previously mentioned, we chose not to take the patient’s airway and the medevac team was well-prepared to intubate in flight.
Serial peak flow measurements could be used to monitor impending respiratory collapse, and intubating pre-emptivelyshould be considered. Approaching this intoxication similarly to a combined pill-based sodium and calcium channel blocker intoxication might offer further treatment options, including intravenous sodium bicarbonate independent of QRS duration, giving a fluid bolus with diuresis to more rapidly evacuate the toxin from the system, and standard treatments for pill-based calcium channel blockade intoxication via poison control including intravenous calcium. Treatment with sodium bicarbonate is indicated for a QRS duration >100 ms. On intake, our patient had a QRS duration of 90 ms and, other than tachycardia, with a rate of 106 beats per minute, there were no other EKG abnormalities initially in the clinic or during the flight until the abrupt arrest. There is no way to ascertain initially whether the PSP patient will have brief paresthesias only, require respiratory support, or abruptly go into full respiratory and cardiac arrest as was the case with our patient. One contaminated mussel can be fatal.
Conclusion
Research is ongoing into the cause of increased toxic algae blooms in the Aleutian Islands, but the exact combination of conditions that cause blooms is not yet known. The current situation of rising temperatures in the Bering Sea appears to coincide with increased algae blooms and concerning toxin measurements throughout the Aleutian Islands and surrounding Alaska.2 Cases of paralytic shellfish poisoning are fortunately rare since the advent of monitoring systems.7-14 Considering the mechanism of paralytic shellfish toxin and future treatment options, a review of sodium channel blocker overdose treatment and calcium channel blocker overdose treatment was conducted.
In a sodium channel blocker overdose, the standard treatment is intravenous sodium bicarbonate. This is traditionally recommended in patients with an EKG QRS duration >100 ms or any suspicious QT prolongation or dysrhythmia.18Intravenous sodium bicarbonate will raise the serum pH and increase extracellular sodium. This alkalinization increases the electrochemical gradient across cell membranes, helping to offload sodium channels. Patients should be given 1-2mEq/kg as a bolus dose.18 Bolus doses can be administered until the QRS duration is <100 ms. This can be followed with a continuous infusion of sodium bicarbonate of 2-3 50mEq ampules in 1 liter of D5W.18 Extracorporeal membrane oxygenation was used in a case of sodium channel blocker overdose that was not responding to treatment and resulted in a survival.19
Calcium channel blocker intoxication causes bradycardia, hypotension, conduction disturbances, and escape rhythms.20 Treatment of a calcium channel blocker intoxication consists of monitoring only in an asymptomatic patient with normal vital signs and a normal EKG; in symptomatic or known high-dose cases, intravenous calcium chloride or calcium gluconate with or without insulin, methylene blue, lipid emulsion, and glucagon. Catecholamines are recommended if severe hypotension.20 Hemodialysis is ineffective against a calcium channel blocker overdose.
In a series of 7 cases, Hurley et al intubated the first patient but monitored the remaining 6 patients with serial peak flow measurements.21 They did not collect height and weight on their patients, so they were unable to calculate values for recommendations.21 Serial peak flow measurements might offer some promise in the future to have an early warning on impending respiratory collapse.
In conclusion, we propose consideration of early intubation, bolus of sodium bicarbonate, calcium, and extracorporeal membrane oxygenation if available. In remote areas with low resources, consideration of intravenous fluid bolus and high-dose diuretic can be made to possibly assist in flushing the toxin out of the system. Consider serial peak flow measurements to assist with an early indication of impending respiratory compromise. Continue life support measuresif there is a chance that the toxin can be flushed out of their system and the patient can survive the intoxication. Paralytic shellfish poisoning is technically reversible if they can survive the paralysis and system shutdown.7-10
Acknowledgements
The authors would like to acknowledge the Alaska State Epidemiological Lab, Environmental Health Laboratory Anchorage, Providence Alaska Hospital, Lifemed Alaska Medevac, Iliuliuk Family Health Service Clinic, Unalaska Fire and Rescue Emergency Medical Services, Chandra Poe, Qawalangin Tribe of Unalaska, Knik Tribe of Alaska, and the United States Environmental Protection Agency.
Author Contributions
Sarah Spelsberg was the attending provider for the patient and lead author. Megan Sarnecki assisted with state laboratory and tribe communication, patient test results compilation and editing. Sara Filmalter assisted with literature review and editing. Abraham Boxx assisted with literature review, writing, and editing. Heather Edmison was the attending paramedic on the Lifemed Alaska Medevac plane for the patient and assisted with paper edits. Holli Marie Carter was the attending nurse on the Lifemed Alaska Medevac plane for the patient and assisted with paper edits.
References
- Wang D.-Z. Neurotoxins from marine dinoflagallates: A brief review. Mar. Drugs. 2008;6:349–371. doi: 10.3390/md6020349. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Paralytic Shellfish Poisoning Fact Sheet. Accessed 04/10/2022. https://dhss.alaska.gov/dph/Epi/id/SiteAssets/Pages/Shellfish%20Poisoning%20Resources/PSP_factsheet.pdf.
- Llewellyn L.E. Predictive toxinology: An initial foray using calculated molecular descriptors to describe toxicity using saxitoxin as a model. Toxicon. 2007;50:901–913. doi: 10.1016/j.toxicon.2007.06.015. [PubMed] [CrossRef] [Google Scholar].
- Cestele S., Catterall W.A. Molecular mechanisms of neurotoxin action on voltage-gated sodium channels. Biochimie. 2000;82:883–892. doi: 10.1016/S0300-9084(00)01174-3. [PubMed] [CrossRef] [Google Scholar].
- Etheridge S.M. Paralytic shellfish poisoning: Seafood safety and human health perspectives. Toxicon. 2010;56:108–122. [PubMed] [Google Scholar].
- Wang D.-Z. Neurotoxins from marine dinoflagallates: A brief review. Mar. Drugs. 2008;6:349–371. doi: 10.3390/md6020349. [PMC free article] [PubMed] [CrossRef] [Google Scholar].
- Van Dolah F.M. Marine algal toxins: Origins, health effects, and their increased occurrence. Environ. Health Perspect. 2000;108:133–141. doi: 10.1289/ehp.00108s1133. [PMC free article] [PubMed] [CrossRef] [Google Scholar].
- Landsberg J.H. The effects of harmful algal blooms on aquatic organisms. Rev. Fish. Sci. 2002;10:113–390. [Google Scholar].
- Food and Agricultural Organization . Marine Biotoxins FAO Food and Nutrition Paper 80. Food and Agricultural Organization of the United Nations; Rome, Italy: 2004. [Google Scholar].
- Stuken A., Orr R.J.S., Kellmann R., Murray S.A., Neilan B.A., Jakobsen K.S. Discovery of nuclear-encoded genes for the neurotoxin saxitoxin in dinoflagellates. PLoS One. 2011;6:e20096. [PMC free article] [PubMed] [Google Scholar].
- Hackett J.D., Wisecarver J.H., Brosnahan M.L., Kulis D.M., Anderson D.M., Bhattacharya D., Plumley F.G., Erdner D.L. Evolution of saxitoxin synthesis in cyanobacteria and dinoflagellates. Mol. Biol. Evol. 2013;30:70–78. doi: 10.1093/molbev/mss142. [PMC free article] [PubMed] [CrossRef] [Google Scholar].
- Llewellyn L.E. Predictive toxinology: An initial foray using calculated molecular descriptors to describe toxicity using saxitoxin as a model. Toxicon. 2007;50:901–913. doi: 10.1016/j.toxicon.2007.06.015. [PubMed] [CrossRef] [Google Scholar].
- Cestele S., Catterall W.A. Molecular mechanisms of neurotoxin action on voltage-gated sodium channels. Biochimie. 2000;82:883–892. doi: 10.1016/S0300-9084(00)01174-3. [PubMed] [CrossRef] [Google Scholar].
- Wang J, Salata JJ, Bennett PB. Saxitoxin is a gating modifier of HERG K+ channels. J Gen Physiol. 2003 Jun;121(6):583-98. doi: 10.1085/jgp.200308812. PMID: 12771193; PMCID: PMC2217357.
- Cusick KD, Sayler GS. An overview on the marine neurotoxin, saxitoxin: genetics, molecular targets, methods of detection and ecological functions. Mar Drugs. 2013;11(4):991-1018. Published 2013 Mar 27. doi:10.3390/md11040991 (NA K AND CA).
- Barbosa V, Santos M, Anacleto P, et al. Paralytic Shellfish Toxins and Ocean Warming: Bioaccumulation and Ecotoxicological Responses in Juvenile Gilthead Seabream (Sparus aurata). Toxins (Basel). 2019;11(7):408. Published 2019 Jul 13. doi:10.3390/toxins11070408.
- Ecosystem Status Report 2020 Eastern Bering Sea. Accessed 04/11/2022. https://apps-afsc.fisheries.noaa.gov/REFM/docs/2020/EBSecosys.pdf.
- Dokken K, Fairley P. Sodium Channel Blocker Toxicity. [Updated 2022 Jul 18]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534844/.
- Vu NM, Hill TE, Summers MR, Vranian MN, Faulx MD. Management of life-threatening flecainide overdose: A case report and review of the literature. HeartRhythm Case Rep. 2016 May;2(3):228-231.
- Chakraborty RK, Hamilton RJ. Calcium Channel Blocker Toxicity. [Updated 2022 Apr 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537147/.
- Hurley W, Wolterstorff C, MacDonald R, Schultz D. Paralytic shellfish poisoning: a case series. West J Emerg Med. 2014 Jul;15(4):378-81. doi: 10.5811/westjem.2014.4.16279. PMID: 25035737; PMCID: PMC4100837.



