Ch. 8 - Neuropathic Pain
Sundeep Jassal, DO | Crozer-Chester Medical Center
Richard Pescatore, DO, FAAEM | Delaware Division of Public Health
Neuropathic pain (NP), often known as the "disease of pain" 1 is a noxious sensation caused by a lesion or disease that directly affects the somatosensory system.2 An estimated 4 million people in the United States are affected by NP3, and approximately 16% of people in the United States are suffering from chronic pain with a neuropathic component.4
Even these figures are believed to be underestimates due to under-eporting and misdiagnosis. Multifactorial influences, varying symptomatology, and a lack of a clear "gold standard" for an NP diagnosis have made it difficult for health care providers to accurately and efficiently diagnose patients.4
Etiology of Neuropathic Pain
Both proper identification and treatment of NP are critical, as the disease process is known to worsen and evolve if not treated appropriately. Changes in sensory signaling at the level of the peripheral nervous system, spinal cord, and the brain (specifically the thalamus and cortex) occur over weeks and months. If left untreated, these changes in normal sensory signaling may lead to permanent changes in both genomic expression and cortical structures.1,5
Common types of NP pathologies include chronic radicular pain, diabetic neuropathies, and complex regional pain syndrome (CPRS).3 NP is also common among patients with human immunodeficiency virus (HIV) secondary to disease progression as well as antiretroviral agents. 3 NP is often seen among cancer patients due to tumor-induced neuronal compression, radiation-induced neuronal injuries, chemotherapeutic agents, and paraneoplastic disorders. 3 Other common causes of NP include postherpetic pain, post-stroke neuropathy, chronic pain syndrome, fibromyalgia with radiculopathy, and phantom limb pain following an amputation.
Signs and Symptoms of Neuropathic Pain
Along with pain, the hallmarks of NP include paresthesias (nonpainful abnormal sensations) and dysesthesias (unpleasant abnormal sensations). Allodynia (pain that is caused by a non-noxious stimulus), hyperalgesia (an increased sense of pain that is caused by a noxious stimulus), spontaneous pain, or electric shock-like "shooting" pain, and burning pain sensations may also be described. 3
Pharmacologic Treatment Options for Neuropathic Pain
Treatment options for NP correspond directly with the mechanisms that cause the disease. Many of the treatment options for NP are based on what we already understand regarding the over-expression and increased activation of sodium (Na+), potassium (K+), and calcium (Ca2+) channels as well as increased activation of NMDA receptors. These understandings have led to critical advancements in the treatment of NP.
Ketamine
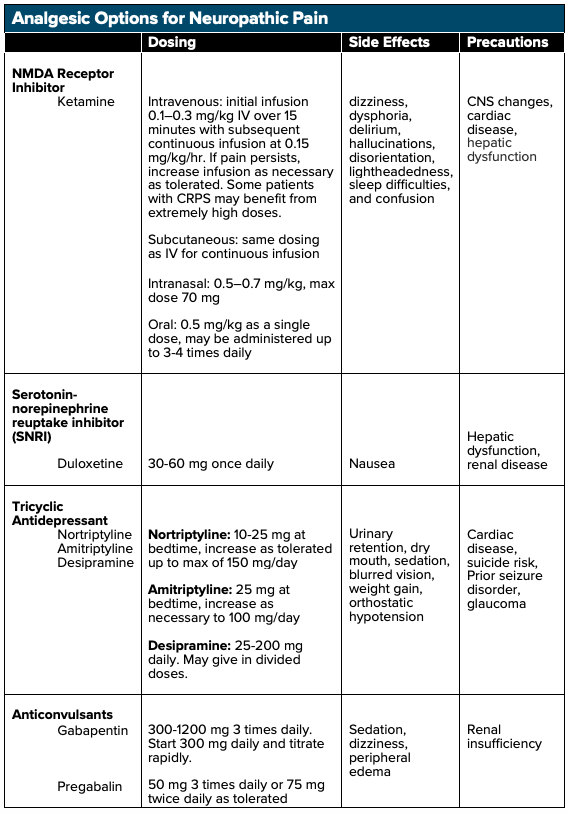
Ketamine, a noncompetitive NMDA and glutamate receptor antagonist, has been shown to be efficacious in the treatment of NP. By blocking the NMDA receptor, ketamine halts continued nonselective cation channel opening and continued excitation, which may aid in halting central sensitization (ie, decrease signaling to the brain).7 Ketamine can be administered intravenously (IV), subcutaneously (SQ), intranasally (IN), sublingually (SL), orally (PO), and topically. Research has shown that ketamine administration at sub-dissociative levels (SDK, 0.1–0.3 mg/kg IV) via short-term and continuous infusions (0.15 mg/kg/hr) results in significant rates of pain reduction when compared to placebo with up to a 50% reduction in NP.8,9 Studies have also demonstrated that ketamine infusions assist in long-term, continued analgesia9 and has shown to be effective in the treatment of NP that have been refractory to other treatment modalities.10 Subcutaneous administration of low-dose ketamine infusions have also been shown to reduce chronic pain refractory to first-line analgesics.11 As with all medications, however, ketamine has adverse side effects including dizziness, dysphoria, hallucinations, disorientation, lightheadedness, and confusion, which can be limited when given as a slow intravenous infusion.12
Antidepressants
Antidepressants such as tricyclic antidepressants (TCAs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) block the reuptake of both norepinephrine as well as serotonin, leading to increases of the hormones within the synaptic cleft. Multiple trials have shown that both TCAs and SNRIs have demonstrable benefits in the treatment of NP when compared to placebo.14 When employed in the treatment of certain NP presentations, the number needed to treat (NNT) for 50% pain relief for TCAs is 3.6 while the NNT for SNRIs is 6.4.15 It is important to note that TCAs may have anticholinergic and antihistamine side effects, which may result in orthostatic hypotension, drowsiness, and dry mouth. A baseline EKG should also be obtained before initiating therapy as TCAs may lead to QT prolongation.15
Gabapentinoids
By directly binding voltage-gated calcium channels, gabapentinoids (gabapentin or pregabalin) prevent the release of calcium ions leading to decreased excitation and signaling and reduced central pain sensitization.16 Both gabapentin and pregabalin have shown efficacy when compared to placebo in the treatment of NP,14 with an NNT for 50% pain reduction with gabapentin of 6.3 and 7.7 for pregabalin.15 It is important to note that both gabapentin and pregabalin are renally-cleared, and lower doses may be required for patients with renal insufficiency.14 Side effects of both gabapentin and pregabalin include dizziness, fatigue and drowsiness, ataxia, peripheral edema, nystagmus, and tremor, with drowsiness and dizziness being the most commonly cited side effects.17,18 Caution should be taken in elderly patients, in which gabapentin can cause or exacerbate cognitive impairments.17 At therapeutic doses, gabapentinoids have been shown to have severe drug-drug interactions and has been shown to potentiate the effects of opioids during concomitant use, leading to an increased risk of abuse.19
Lidocaine
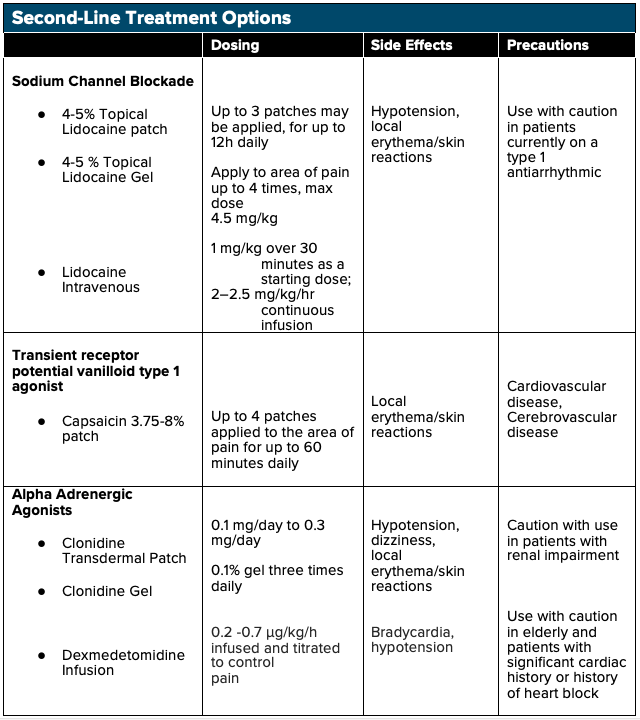
IV lidocaine may be effective in the treatment of NP as a sodium channel blocking agent. Lidocaine is effective in inhibiting both peripheral nociceptor’s sensitization and central hyperexcitability.20 In addition to its sodium channel blocking effects, lidocaine also exhibits anti-inflammatory properties - reducing circulating cytokines - which play a crucial role in preventing secondary hyperalgesia and central sensitization.20 Multiple studies have shown the efficacy of lidocaine infusions in the treatment of NP, including spinal cord injury-associated NP, diabetic neuropathy, and even chronic pain syndromes such as fibromyalgia. The majority of these studies have shown that higher dose IV lidocaine infusions (5 mg/kg) have shown more significant effects in pain modulation as compared to lower dose lidocaine infusions (1 mg/kg).20 However, adverse cardiovascular effects can take place with higher doses, and further research with the administration in the emergency setting is needed before IV lidocaine is to be considered a first-line treatment for NP.
Clonidine
As an alpha-2 adrenergic receptor agonist, clonidine is commonly used as an antihypertensive, and it has also been shown to have a role in the treatment of acute pain. Recent studies have shown that clonidine may have efficacy in the treatment of chronic pain syndrome as well.21 When used as an adjunct treatment with opioids for NP, the combination has shown a greater reduction than either drug used alone. 21
Dexmedetomidine
Dexmedetomidine is an alpha-2 adrenergic receptor agonist often used for short term sedation in ICU settings but recently has shown a possible role in chronic pain control. DXMT functions as a presynaptic α-2-adrenergic receptor agonist in the locus coeruleus, dampening the sympathetic response and inducing sedation, anxiolysis, and analgesia. 22,23 DXMT has demonstrated efficacy in several case studies when used in the treatment of chronic postoperative pain in patients who were unable to tolerate opioids due to opioid-induced hyperalgesia and has shown to reduce overall opioid requirements in these patients as well. 24 However, limited data is available to support DXMT use for NP in the emergency setting, and further research is needed to assess the safety and efficacy of its use.
Topical Analgesia
Patients experiencing more peripheral symptoms from their NP may benefit from topical analgesics such as 5% lidocaine patch, EMLA cream, and capsaicin cream. Topical lidocaine patches function via sodium channel blockade, thus inhibiting neuronal firing. As opposed to PO and IV analgesics, which require systemic distribution, topical/transdermal analgesics use localized analgesic distribution to limit total-body quantities delivered.25,26 Topical NSAIDs can preferably accumulate in targeted areas such as cartilage and meniscus with concentrations 4-7 times greater than plasma concentrations, and in tendons with concentrations one hundred times greater than plasma concentrations.26 Topical analgesics are optimal in patients with renal disease or elderly patients susceptible to elevated analgesic plasma concentrations and in patients with multiple comorbidities such as peptic ulcer disease and cardiovascular disease in which oral NSAIDs are relatively contraindicated.27 Topical analgesics are a relatively affordable option for patients and have shown some benefit versus placebo. Lidocaine patches should be used with caution in patients on class 1 antiarrhythmic agents.15
Similarly, topical capsaicin patches have some proven benefit in the treatment of NP, with an NNT of 10.6 for a 50% reduction of pain. Capsaicin directly activates transient receptor potential vanilloid 1 (TRPV1) ligand-gated channels on nociceptive fibers, ultimately causing desensitization of TRPV1 and inhibiting nerve fibers within the epidermis. Patients have reported some pain and skin discomfort with the application. However, studies have shown that one single application of high-concentration formulations (office use only; not available for prescription) for 30-60 minutes may have effects lasting up to 3 months.15
Opioids
Once considered first-line treatment options for NP, opioids have fallen to second-line treatment in the management algorithm. Multiple RCTs have demonstrated opioids to have similar efficacy in the treatment of NP as first-line treatments, but with the apparent risks of addiction and overdose.14 Though opioids may provide the most immediate short-term pain relief to patients, up to 50% of patients treated with opioids face addiction, abuse, or misuse.14 Opioids such as morphine or oxycodone/hydrocodone may be more appropriate as an adjunct to first-line therapies for NP, in specific cases of cancer-induced NP, and in cases of acute pain presentations in patients with chronic NP pain. It is important to remember that opioids can induce a rebound effect in some patients resulting in opioid-induced hyperalgesia, a paradoxical response in which patients may become increasingly sensitive to painful stimuli.28
Non-Pharmacologic Treatment Options for Neuropathic Pain
Acupuncture. Multiple nonpharmacologic options have been used for the treatment of NP. Acupuncture, an ancient technique of needling in the skin and dermis, was developed in ancient China and has often been used in Western medicine for the treatment of NP. A Cochrane systematic review found little evidence that acupuncture is more efficacious than a placebo.29 Some recent studies, however, have suggested that patients may have increased quality of life and a decrease in their pain intensity after having acupuncture. 29 However, rigorous trials with hard endpoints to assess the risks and benefits to acupuncture therapy are lacking. At this time, further research is needed to assess the safety and efficacy of acupuncture in the emergency setting.
Osteopathic manipulative therapy (OMT) is another non-invasive, non-pharmaceutical option that patients may attempt for the treatment of their NP. One trial investigating OMT demonstrated alterations in the release of endogenous biomarkers, including opioids and serotonin. 30 Studies have suggested that when combined with pharmacotherapy, OMT may provide significant efficacy in the treatment of NP and improvement of quality of life.31 As with acupuncture, however, most trials suggesting a benefit to OMT utilize unvalidated surrogate outcomes, with no high-quality investigation to date demonstrating benefit and should be trialed outside the emergency setting. Further research is needed to assess the safety and efficacy of acupuncture in the emergency setting.
Stepwise Treatment Pathway for Neuropathic Pain in the Emergency Setting
Step 1
- Assess patient, past medical history, comorbidities, and their pain, use these measures as a guide in establishing the diagnosis of NP and the likely etiology of the patient's pain
- Based on the patient's comorbidities and previous analgesic regimen (following discussion with a pain specialist), identify a treatment plan that focuses on limiting factors such as renal disease and cardiovascular disease.
- Provide a referral for patients to a pain specialist or a neurologist if there is uncertainty regarding the diagnosis of NP.
Step 2
- Based on the etiology of the patient's NP, initiate therapy:
- Begin therapy with a first-line agent
- NMDA Receptor Inhibitor (Ketamine)
- Tricyclic Antidepressant (TCA)
- Serotonin-norepinephrine reuptake inhibitor (SNRI)
- Gabapentinoid
- IV Lidocaine
- If symptoms persist on first-line monotherapy consider combination therapy
- TCA + Gabapentinoid
- SNRI + Gabapentinoid
- Dexmedetomidine
- Consider opioid analgesics in cases of NP caused by breakthrough cancer pain, acute exacerbations not otherwise controlled by other first-line agents, or for severe refractory pain
- Morphine
- Hydrocodone
- Oxycodone
- For patients experiencing peripheral NP, consider second-line topical agents
- 4-5% lidocaine patches
- 4-5% topical lidocaine gel
- 3.75-8% capsaicin patch
- Begin therapy with a first-line agent
Step 3
- Reassess the patient's pain, symptoms, and quality of life on current therapy and determine if pain and symptoms appear to be well-controlled
- If patients seem to have some relief but are still suffering from NP, consider combination therapy if on monotherapy or an alternative combination therapy if already on multiple first-line agents
- If the patient has no relief of their symptoms from their initial first-line treatment consider an alternative first-line agent (and alternative sources of pain)
Step 4
- If the patient's pain persists despite the above therapies refer them to a pain specialist for further care; admission may be required in extreme cases
- Offer outpatient non-pharmaceutical therapy recommendations
- Osteopathic manipulation
- Acupuncture
- Exercise
- Alternating cold and hot packs
References
- Alles S, Smith P. Etiology and Pharmacology of Neuropathic Pain. Pharmacol Rev. 2018;70(2):315-347.
- IASP. Neuropathic Pain. 2019.
- Chen H, et al. Contemporary management of neuropathic pain for the primary care physician. Mayo Clin Proc. 2004;79(12):1533-45.
- DiBonaventura MD, et al. The prevalence of probable neuropathic pain in the US: results from a multimodal general-population health survey. J Pain Res. 2017;10:2525-2538.
- Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1-32.
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10(9):895-926.
- Niesters M, Martini C, Dahan A. Ketamine for chronic pain: risks and benefits. Br J Clin Pharmacol. 2014;77(2):357-67.
- Cohen SP, et al. Consensus Guidelines on the Use of Intravenous Ketamine Infusions for Chronic Pain From the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018;43(5):521-546.
- Noppers I, et al. Ketamine for the treatment of chronic non-cancer pain. Expert Opin Pharmacother. 2010;11(14):2417-29.
- Hana Z, Abdulla S, Alam A, Ma D. Ketamine: Old Drug But New Use for Neuropathic Pain. Translational Perioperative and Pain Medicine. 2018. doi:10.31480/2330-4871/062.
- Zekry O, Gibson SB, Aggarwal A. Subanesthetic, Subcutaneous Ketamine Infusion Therapy in the Treatment of Chronic Nonmalignant Pain. J Pain Palliat Care Pharmacother. 2016;30(2):91-8.
- Subramaniam K, Subramaniam B, Steinbrook RA. Ketamine as adjuvant analgesic to opioids: a quantitative and qualitative systematic review. Anesth Analg. 2004;99(2):482-95.
- Obata H. Analgesic Mechanisms of Antidepressants for Neuropathic Pain. Int J Mol Sci. 2017;18(11):2483.
- O'Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med. 2009;122(10 Suppl):S22-32.
- Attal N, et al. Chronic neuropathic pain management in spinal cord injury patients. What is the efficacy of pharmacological treatments with a general mode of administration? (oral, transdermal, intravenous). Ann Phys Rehabil Med. 2009;52(2):124-41.
- Fornasari D. Pharmacotherapy for Neuropathic Pain: A Review. Pain Ther. 2017;6(Suppl 1):25-33.
- Kremer M, Salvat E, Muller A, Yalcin I, Barrot M. Antidepressants and gabapentinoids in neuropathic pain: Mechanistic insights. Neuroscience. 2016;338:183-206.
- Markman JD, Dworkin RH. Ion channel targets and treatment efficacy in neuropathic pain. J Pain. 2006;7(1 Suppl 1):S38-47.
- Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction. 2016;111(7):1160-74.
- Kandil E, Melikman E, Adinoff B. Lidocaine Infusion: A Promising Therapeutic Approach for Chronic Pain. J Anesth Clin Res. 2017;8(1):697.
- Kumar A, Maitra S, Khanna P, Baidya DK. Clonidine for management of chronic pain: A brief review of the current evidences. Saudi J Anaesth. 2014;8(1):92-96.
- Mahmoud M, Mason KP. Dexmedetomidine: review, update, and future considerations of paediatric perioperative and periprocedural applications and limitations. Br J Anaesth. 2015;115(2):171-82.
- Cozzi G, Monasta L, Maximova N, Poropat F, Magnolato A, Sbisa E, et al. Combination of intranasal dexmedetomidine and oral midazolam as sedation for pediatric MRI. Paediatr Anaesth. 2017;27(9):976-7.
- Belgrade M, Hall S. Dexmedetomidine Infusion for the Management of Opioid-Induced Hyperalgesia. Pain Med. 2010;11(12):1819-1826.
- Leppert W, et al. Transdermal and Topical Drug Administration in the Treatment of Pain. Molecules. 2018;23(3).
- McCarberg B, D'Arcy Y. Options in topical therapies in the management of patients with acute pain. Postgrad Med. 2013;125(4 Suppl 1):19-24.
- American College of Rheumatology Ad Hoc Group on Use of, S. and D. Nonselective Nonsteroidal Anti-inflammatory, Recommendations for use of selective and nonselective nonsteroidal anti-inflammatory drugs: an American College of Rheumatology white paper. Arthritis Rheum. 2008;59(8):1058-73.
- Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician. 2011;14(2):145-161.
- Ju ZY, et al. acupuncture for neuropathic pain in adults. Cochrane Database Syst Rev. 2017;12:CD012057.
- Degenhardt BF, et al. Role of osteopathic manipulative treatment in altering pain biomarkers: a pilot study. J Am Osteopath Assoc. 2007;107(9):387-400.
- Arienti C, et al. Osteopathic Manipulative Treatment Effect on Pain Relief and Quality of Life in Oncology Geriatric Patients: A Nonrandomized Controlled Clinical Trial. Integr Cancer Ther. 2018;17(4):1163-1171.
- Sansone RA, Sansone LA. Tramadol: seizures, serotonin syndrome, and coadministered antidepressants. Psychiatry (Edgmont). 2009;6(4):17-21.
- Delage N, et al. Effect of Ketamine combined with magnesium sulfate in neuropathic pain patients (KETAPAIN): study protocol for a randomized controlled trial. Trials. 2017;18(1):517.
- Sun W, et al. Oxytocin Relieves Neuropathic Pain Through GABA Release and Presynaptic TRPV1 Inhibition in Spinal Cord. Front Mol Neurosci. 2018;11:248.
- Kimura M, Saito S, Obata H. Dexmedetomidine decreases hyperalgesia in neuropathic pain by increasing acetylcholine in the spinal cord. Neuroscience Letters. 2012;529(1):70-74.





