Ch. 10.5 - Buprenorphine Initiation in the ED and MAT
David H. Cisewski, MD, MS | Icahn School of Medicine at Mount Sinai
Reuben J. Strayer, MD | Maimonides Medical Center
BACKGROUND
Against the backdrop of exploding opioid prescriptions in the late 1990s and early 2000s, the medical community has not only recognized the addictive power of opioids but has also witnessed the emergence of a nationwide opioid use disorder (OUD) epidemic affecting individuals of every gender, race, and socioeconomic status. Current estimates suggest three million individuals in the United States have suffered from OUD.1
As with any disease, it is the responsibility of emergency clinicians to recognize individuals suffering from OUD and provide or arrange for appropriate treatment for patients desiring assistance.
Buprenorphine is a medication for opioid use disorder (MOUD) which, when adhered to, safely and effectively transitions people with OUD to recovery. This chapter will provide an overview of buprenorphine, the benefits of treating OUD with buprenorphine in the emergency department (ED), and how emergency clinicians can effectively utilize buprenorphine for patients presenting to the ED with OUD.
WHAT IS BUPRENORPHINE?
Buprenorphine is a semisynthetic derivative of thebaine, developed in 1978 to treat chronic pain conditions. In the United States, buprenorphine is approved to treat OUD and moderate/severe chronic pain.2,3
In contrast to full agonist opioids such as fentanyl, morphine, hydromorphone, oxycodone, methadone, and heroin, buprenorphine is a partial mu opioid receptor agonist with a very high receptor affinity (preferential binding), low intrinsic receptor activation (partial agonism), and slow rate of dissociation (extended duration). These three qualities make buprenorphine uniquely suited as a treatment for OUD.
When administered to a person experiencing opioid withdrawal syndrome (OWS), buprenorphine abolishes withdrawal symptoms, and in sufficient doses, extinguishes the persistent cravings that lead to hazardous return to use (relapse). Because of its partial agonism, and in contrast to every other commonly used opioid, buprenorphine’s capacity to cause respiratory depression is limited by a ceiling effect – buprenorphine is comparatively very safe in overdose and far safer than the primary MOUD alternative, methadone. Partial agonism also blunts buprenorphine’s euphoric potential, making it less likely to be abused than full agonist opioids.4 The high receptor affinity blocks co-administered opioids from binding, effectively limiting the risk of overdose or the euphoria of full agonist opioids while a patient is therapeutic on buprenorphine; this is called buprenorphine blockade.
The slow dissociation of buprenorphine from the receptor allows for an extended half-life ranging from between 6-12 hours for low doses (< 4 mg) and between 24-72 hours (or even longer) for higher doses (> 16 mg), which allows for convenient sublingual daily dosing.5
Beyond the commonly used sublingual tablets and strips, buprenorphine comes in an expanding array of formulations that include an injectable liquid for intravenous or intramuscular use, a transdermal patch, and a variety of depot and implantable preparations that offer weekly, monthly, or even longer dosing schedules.
Sublingual buprenorphine is available in its original mono-product formulation, but when treating OUD is usually prescribed as a combination product, buprenorphine-naloxone, and buprenorphine is best known by the trade name of the first buprenorphine-naloxone preparation, Suboxone. It is commonly taught that the naloxone component of buprenorphine-naloxone is not absorbed when taken under the tongue as intended, and that the presence of naloxone serves only to prevent abuse by crushing and injecting the medication. However, this teaching has recently been called into question, as naloxone is absorbed sublingually6 and in some patients causes mild but sometimes quite bothersome antagonist effects, and the abuse-deterrent effect of the naloxone additive is modest at best.7,8 Furthermore, diverted buprenorphine is usually used for its intended purpose,9,10 and while injecting buprenorphine exposes the user to dangerous injection harms, it is far safer than injecting street-purchased alternatives of uncertain identity and potency.
ADVERSE EFFECTS OF BUPRENORPHINE
Like all opioids, buprenorphine can result in sedation and respiratory depression when consumed at increasing doses. Though buprenorphine is unlikely to cause dangerous respiratory depression in otherwise healthy adults when taken even in very high doses, harmful toxicity may occur when buprenorphine is used in combination with other sedatives (eg, alcohol, benzodiazepines), in the very young, the very old, or the frail.11,12
Additionally, when buprenorphine is administered to an opioid-dependent person who is not in spontaneous withdrawal (ie, has a therapeutic level of full-agonist opioid in their system), buprenorphine, because of its high receptor affinity, will displace the full agonist on the receptors. As buprenorphine is a partial agonist displacing a full agonist, the patient in this case suffers a loss of agonist and will start to withdraw. This is called buprenorphine-precipitated withdrawal (BPW), and though sometimes attributed to the naloxone component of bup-nalox combination, it is actually a pharmacologic feature of the buprenorphine itself.13,14 BPW is more likely to occur when buprenorphine is administered to patients with low COWS scores (see below), patients on methadone, or patients who have been using fentanyl for a prolonged period (often unknowingly), as fentanyl accumulates and is slowly leached from fat stores.15 The risk of BPW is reduced by assuring that the patient has a high COWS score before initiating treatment. Though evidence is scant, it is likely that the best treatment of BPW is higher doses of buprenorphine under close observation.16
BUPRENORPHINE USE IN THE ED
Any patient presenting to the ED with suspected or confirmed OUD - especially experiencing OWS - who is not already enrolled in a medication-based treatment program (buprenorphine, methadone, or naltrexone), should be considered a candidate for buprenorphine treatment. The more the patient is being harmed by their opioid use, the greater the potential benefit of buprenorphine. Given that the emergency department is the only access that many people with OUD have to medical care, emergency providers are uniquely positioned to intervene on this highly prevalent, lethal, and treatable disease. ED-initiated buprenorphine has been associated with increased engagement in outpatient opioid addiction treatment programs, as well as reduced illicit opioid use in those who continued the treatment transition to outpatient care.17
Specific Populations
- Pregnant/Breastfeeding patients. Buprenorphine is safe during pregnancy, and a 2017 American Congress of Obstetricians and Gynecologists (ACOG) opinion statement supported the use of buprenorphine during pregnancy to treat OUD.18 Additionally, both ACOG and the American Academy of Pediatrics (AAP) support buprenorphine use for OUD during breastfeeding, regardless of the maternal dose.18,19 Despite traditional teaching, either the buprenorphine monoproduct or buprenorphine/naloxone combination may be used in pregnancy.
- "Fully detoxed" patients. Patients who have completed a period of opioid detoxification and are no longer suffering withdrawal symptoms can still experience debilitating cravings, which lead to very dangerous relapse. Buprenorphine should be initiated without concern for BPW (as the patient is no longer opioid-dependent) but with consideration of reduced opioid tolerance developing after 1-2 weeks of abstinence, which results in lower buprenorphine doses needed for equal effect. Consider starting at a lower initial dose (2-4 mg sublingual) with titration as required based on patient response.
HOW DO YOU RECOGNIZE OPIOID WITHDRAWAL SYNDROME (OWS)?OWS refers to the constellation of distressing symptoms associated with too-rapid decrease or cessation of opioid use following the development of opioid dependence. Although typically not life-threatening, OWS results in severe pain, dysphoria, and cravings that can lead to desperate behavior and self-treatment with illicit opioids, as is often seen among individuals with OUD.20 Additional signs and symptoms of OWS include tachycardia, dilated pupils, yawning, tremors, rhinorrhea, goose-flesh skin, and diaphoresis, as well as muscle/joint aches, restlessness, anxiety/irritability, and gastrointestinal distress with abdominal pain, vomiting, and diarrhea.21 (see MDCALC or another tool for a COWS calculation)
HOW TO INITIATE BUPRENORPHINE IN THE EMERGENCY SETTING
When treating OUD with buprenorphine, the primary concern is administering the drug too early and causing buprenorphine-precipitated withdrawal. When the patient is in florid withdrawal, a more detailed assessment may not be necessary; however, when unsure, quantify withdrawal severity using the COWS score. The higher the score, the less likely the patient will develop BPW. We generally recommend waiting until the score is >7 or higher if the patient has recently used methadone or is likely to have been using fentanyl. When in doubt, a focus on objective signs of withdrawal such as tachycardia, tremors, diaphoresis, runny nose, and gooseflesh skin.
Once OWS has been established, buprenorphine can be administered using a stepwise approach. Figure 1 offers steps and dosing recommendations based on expert consensus as presented in the 2020 AAEM White Paper Recommendations on Managing Opioid Use Disorder.22
Figure 1. Emergency department initiation of buprenorphine for opioid use disorder
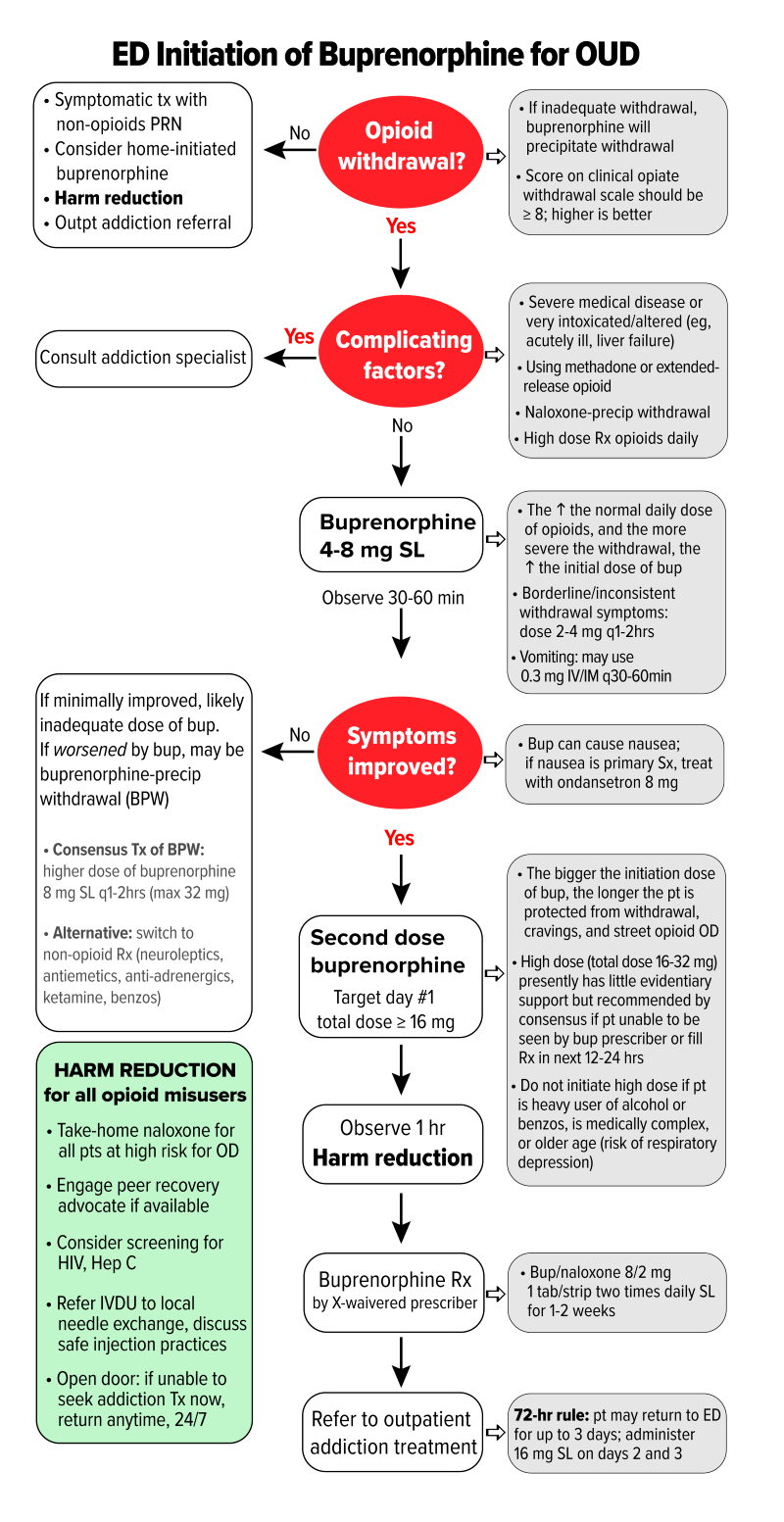
COWS = Clinical Opiate Withdrawal Scale; SL = sublingual; IM = intramuscular; ED = emergency department; OD = overdose; HIV = human immunodeficiency virus; IVDU = intravenous drug user; BID = twice a day. (Figure adapted with permission from authors of the 2020 AAEM White Paper on Opioid Use Disorder in the Emergency Department)
ED DISPOSITION FOLLOWING BUPRENORPHINE INITIATION
The majority of patients presenting to the ED with OWS can be safely discharged with return precautions and close outpatient follow-up. Under the Drug Addiction Treatment Act of 2000 (DATA 2000), physicians must complete eight hours of training and receive an X-waiver qualification to prescribe buprenorphine. However, administration in the ED can be done by any licensed provider. An initial prescription for 16 mg/day (one buprenorphine 8 mg sublingual tab or one buprenorphine/naloxone 8/2 mg sublingual tablet or strip BID for 1-2 weeks) can be provided as a bridge to outpatient management. If hospital regulations permit, consider discharging the patient with a bridging dose of buprenorphine (8-16 mg) to prevent withdrawal in patients at risk of prescription filling delays. Patients should be advised to avoid using sedatives (alcohol, benzodiazepines) while using buprenorphine. However, OUD patients who also have alcohol use disorder or benzodiazepine use disorder should still be treated with buprenorphine.
Patients should be linked to comprehensive outpatient addiction care with the highest level of arrangement possible in the local environment. Follow-up should occur as soon as feasible, ideally with a warm handoff where the emergency provider communicates with the outpatient provider to facilitate the transition of care. The lack of robust follow-up should not dissuade emergency providers from treating patients with buprenorphine, however.
A listing of national buprenorphine treatment practitioners can be found using the SAMHSA practitioner locator. Alternatively, patients can register to an anonymous buprenorphine provider matching system that facilitates patient-provider contact (via www.treatmentmatch.org).
Patients should also be provided with educational material on buprenorphine management and literature on opioid addiction and recovery, if possible. Efforts to ensure patients have access to local pharmacy prescription pickup will increase the likelihood of success. A listing of local pharmacies with buprenorphine availability can be found at the SAMHSA website. Discuss safe household storage tips, especially if the patient lives with children or adolescents.
Take-home naloxone kits should, if possible, be provided to all patients at risk for opioid overdose as well as the friends and family of such patients. Patients should be offered screening for pregnancy, hepatitis C, human immunodeficiency virus, and frank discussions around safe injection practices and needle exchange programs will also assist in preventing disease transmission.
Lastly, advise the patient to return to the ED for further treatment and evaluation if unable to establish outpatient treatment. Emergency clinicians may administer buprenorphine for up to three consecutive days ("72-hour rule") while the patient establishes outpatient treatment.23
Acknowledgment: This chapter is based on the 2020 White Paper recommendations prepared for the American Academy of Emergency Medicine (AAEM). Click here for the full article and a complete discussion on the Management of Opioid Use Disorder in the Emergency Department.
REFERENCES
- Soyka M. New developments in the management of opioid dependence: focus on sublingual buprenorphine-naloxone. Subst Abuse Rehabil. 2015;6(6):1-14.
- Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014(2): p. CD002207.
- Gowing L, Ali R, White JM, Mbewe D. Buprenorphine for managing opioid withdrawal. Cochrane Database Syst Rev. 2017;2:CD002025.
- Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther. 1994;55(5):569-580.
- Khanna IK, Pillarisetti S. Buprenorphine - an attractive opioid with underutilized potential in treatment of chronic pain. J Pain Res. 2015;8:859-870.
- Strickland DM, Burson JK. Sublingual Absorption of Naloxone in a Large Clinical Population. J Drug Metab Toxicol. 2018;9:2.
- Kelty E, Cumming C, Troeung L, Hulse G. Buprenorphine alone or with naloxone: Which is safer? J Psychopharmacol. 2018;32(3):344-52.
- Blazes CK, Morrow JD. Reconsidering the Usefulness of Adding Naloxone to Buprenorphine. Front Psychiatry. 2020;11:549272.
- Chilcoat HD, Amick HR, Sherwood MR, Dunn KE. Buprenorphine in the United States: Motives for abuse, misuse, and diversion. J Subst Abuse Treat. 2019;104:148-157.
- Carlson RG, Daniulaityte R, Silverstein SM, Nahhas RW, Martins SS. Unintentional drug overdose: Is more frequent use of non-prescribed buprenorphine associated with lower risk of overdose? Int J Drug Policy. 2020;79:102722.
- Davies D. Buprenorphine versus methadone--safety first? Br J Gen Pract. 2005;55(512):232-233.
- Megarbane B, Marie N, Pirnay S, et al. Buprenorphine is protective against the depressive effects of norbuprenorphine on ventilation. Toxicol Appl Pharmacol. 2006;212(3):256-267.
- Walsh SL, Eissenberg T. The clinical pharmacology of buprenorphine: extrapolating from the laboratory to the clinic. Drug Alcohol Depend. 2003;70(2 Suppl):S13-S27.
- Hammig R, Kemter A, Strasser J, et al. Use of microdoses for induction of buprenorphine treatment with overlapping full opioid agonist use: the Bernese method. Subst Abuse Rehabil. 2016;7:99-105.
- Rosado J, Walsh SL, Bigelow GE, Strain EC. Sublingual buprenorphine/naloxone precipitated withdrawal in subjects maintained on 100mg of daily methadone. Drug Alcohol Depend. 2007;90(2-3):261-269.
- Oakley B, Wilson H, Hayes V, Lintzeris N. Managing opioid withdrawal precipitated by buprenorphine with buprenorphine. Drug Alcohol Rev. 2021.
- D’Onofrio G, Chawarski MC, O’Connor PG, et al. Emergency Department-Initiated Buprenorphine for Opioid Dependence with Continuation in Primary Care: Outcomes During and After Intervention. J Gen Intern Med. 2017;32(6):660-666.
- ACOG. Opioid Use and Opioid Use Disorder in Pregnancy. Clinical Guidance & Publications 2017 June 19, 2018.
- Sachs HC, Committee On Drugs. The transfer of drugs and therapeutics into human breast milk: an update on selected topics. Pediatrics. 2013;132(3):e796-809.
- Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs. 2003;35(2):253-259.
- White LD, Hodge A, Vlok R, Eastern K, Melhuish TM. Efficacy and adverse effects of buprenorphine in acute pain management: systematic review and meta-analysis of randomised controlled trials. Br J Anaesth. 2018;120(4): 668-678.
- AAEM. Management of Opioid Use Disorder in the Emergency Department: A White Paper Prepared for the American Academy of Emergency Medicine. 2020.
- U.S. Department of Justice Drug Enforcement Administration. Emergency Narcotic Addiction Treatment.





