Ch. 16 - Ultrasound-Guided Nerve Blocks
Arun Nagdev, MD | Highland Hospital/Alameda Health System
Graham Becherer-Bailey, DO | Highland Hospital/Alameda Health System
Robert A. Farrow II, DO, MS | Highland Hospital/Alameda Health System and Mount Sinai Medical Center
Daniel Mantuani, MD, MPH | Highland Hospital/Alameda Health System
Ultrasound-guided nerve blocks (UGNBs) are a vital part of the multimodal approach to the treatment of acute pain in the emergency department. The classic mono-modal approach, which has been the cornerstone of emergency medicine training, has been shown to produce inadvertent acute effects (hypotension, apnea, delirium, etc.) and chronic conditions (opioid addiction) as downstream consequences.
With ultrasound-guidance, these nerve blocks can allow the clinician a more nuanced and patient-tailored approach to pain management. With some education and hands-on practice, ultrasound-guided nerve blocks can become an integral aspect of the emergency physician’s analgesic armamentarium.
Nerve Block Safety & Technique
As with any procedure in medicine, it is essential to consider the necessary steps to safely and effectively perform a nerve block, foresee any complications, and know what steps can be performed to maximize safety in the event of an adverse outcome.
Pre-Block Set Up
Before performing a USGNB, the emergency clinician should identify the area of pain and review potential exclusion criteria, including coagulopathy, anesthesia allergies, or history of neurologic deficiency. Consent should be attained following a discussion of the risks and benefits of the procedure, and a full neurologic exam (including the presence of pulses) should be documented in the patient’s medical record prior to the nerve block. If a fracture is present or and orthopedic intervention may be needed, discuss the block with the orthopedic consult to ensure it doesn’t change their management. Have the patient on a cardiac monitor to assess for systemic toxicity and know where lipid emulsion is available should it be necessary. The provider should mark the extremity and location of the block, and confirm dose administered with two-person verification. (Example use calculation: 1% lidocaine = 1 g/100 mL = 10 mg/mL; 7 mg/kg x 70 kg patient = 490 mg; 490 mg @ 10 mg/mL = 49 mL 1% lidocaine). Lastly, do not forget to perform and document a time out!
For all ultrasound-guided nerve blocks (USGNB), we recommend that the patient be placed on a monitor and have intravenous access in the event further resuscitative efforts are needed following an adverse event. Additionally, because many blocks require high volumes (greater than 20 cc of anesthetic), we prefer a two-person technique in which one provider advances the needle tip, and the other injects the local anesthetic (LA) through an attached catheter. This technique allows for initial normal saline (NS) injection to hydrodissect the tissue/fascial plane (fig 1), followed by the deposition of anesthetic into the opened fascial plane to visualize injection.
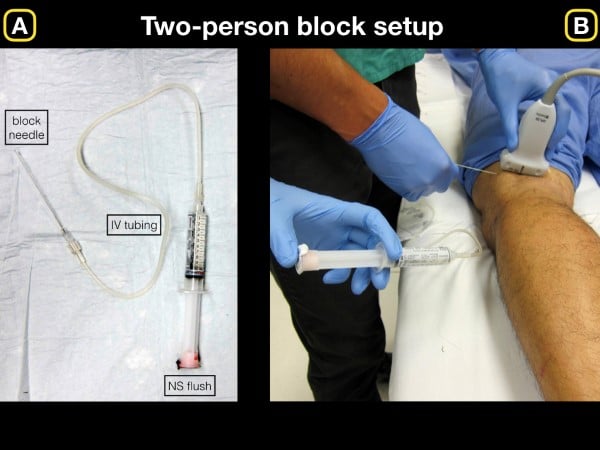
Figure 1. A) Basic setup for initial normal saline injection for block hydrodissection. B) Once the anechoic fluid is injected and clearly visualized on the ultrasound screen, we recommend removing syringe and slowly injecting anesthetic
We recommend using a short, beveled ultrasound-guided nerve block needle if possible (or a more commonly stocked 20-22-gauge Quincke lumbar puncture needle) for the blocks listed below. Until familiar with LA dosing, we advocate referring to readily available weight-based dosing charts for commonly used LAs over a range of patient weights. (See an example here: http://highlandultrasound.com/med-guide). See Table 1 for a list commonly used anesthetics in the emergency setting.
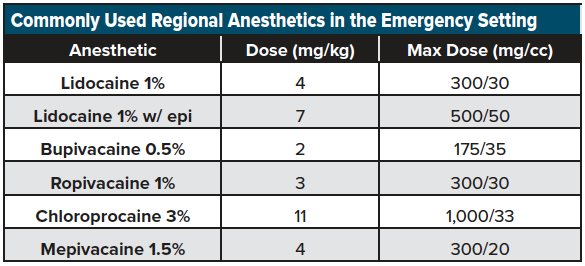
Practitioners performing UGNBs must be familiar with the signs and symptoms of local anesthetic systemic toxicity (LAST) and have lipid emulsion (20%) readily available to treat this rare but life-threatening complication.
Nerve Block Adverse Effects
Although safe when performed using sterile technique and with the safety techniques listed above, nerve blocks come with risks that must be weighed against the analgesic benefit. Each nerve block carries the risk of infection, hematoma, nerve damage, and local anesthetic toxicity if an excess amount is injected directly into the bloodstream. Though rare, patients may develop local anesthesia systemic toxicity (LAST) secondary to direct injection of anesthetics into the vasculature. Adverse effects of local anesthetic toxicity include confusion, anxiety, sense of impending doom, headache, drowsiness, dizzy/lightheadedness, tremors, as well as hemodynamic collapse, widened PR, QRS prolongation and carry the potential for ventricular tachycardia, ventricular fibrillation, hypotension, and asystole. Treatment of LAST includes benzodiazepines for seizures and 1.5 cc/kg bolus IV fat emulsion (intralipid, 20% solution) infused over 1 minute (which can be repeated with the patient started on an infusion if needed – see http://www.lipidrescue.org).
Post-Block Evaluation
Following the completion of the nerve block, repeat the neurologic exam and to write a patient procedure note, which includes the amount of anesthesia used, location of the block, time of administration, and any potential complications associated with the block.
Ultrasound-Guided Nerve Blocks
As discussed, nerve blocks are a simple-to-learn, non-opioid analgesic technique that can result in optimal pain management for the acutely injured patient. Nerve blocks are now seen as an ideal analgesic adjunct, particularly among the elderly population, where the adverse effects of opioids such as sedation, respiratory depression, and inpatient delirium can be minimized with a multimodal analgesic approach that includes a nerve block. Although there are a wide variety of nerve blocks used by anesthetists and pain management specials, there is a core group of blocks available for common emergency department pain presentations. See table 2 for a list of commonly performed ultrasound-guided regional nerve blocks performed in the emergency setting.
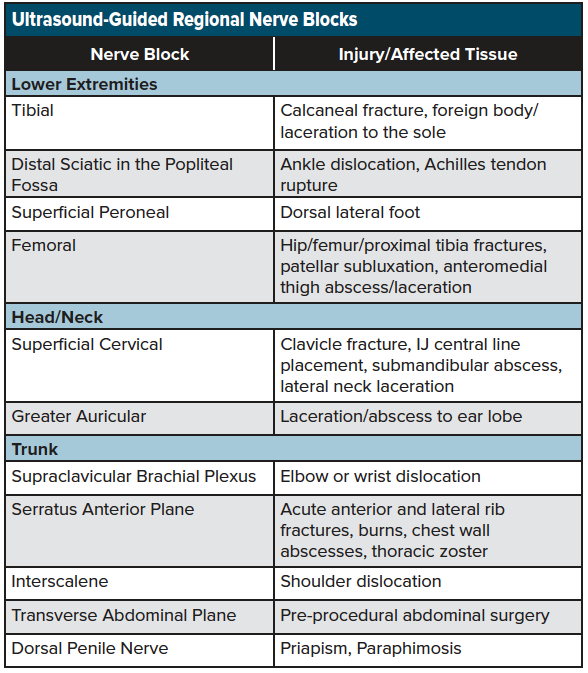
Each of the blocks listed above can be employed using the same systematic approach listed above. Three common blocks will be discussed in this chapter, which are commonly used in many emergency departments and can be integrated into the management of the acutely injured patient.
Femoral Nerve Block
Indication
The ultrasound-guided femoral nerve block (UGFNB) is an ideal adjunct in the emergency department (ED) treatment of femoral neck, intertrochanteric and shaft fractures. This block will also provide analgesia for large thigh lacerations, abscesses, and even some aspect of knee injuries. Hip fractures are particularly ideal for performing a UGFNB, since most patients with this injury pattern are elderly, and commonly inadequately dosed with intravenous opioids secondary to the fear of inadvertent apnea, hypotension, etc.1-4
Anatomy
The femoral nerve is a major branch of the lumbar plexus arising from the 2nd through 4th lumbar ventral rami (L2-4) before descending into the lower extremity over the iliopsoas muscle. The fascial plane overlying the iliopsoas muscle (fascia iliaca) serves as a critical landmark when performing an ultrasound-guided block. The obturator nerve also originates at the L2-4 ventral rami but descends more medially. Due to this medial location, the obturator nerve is variably blocked by the UGFNB.
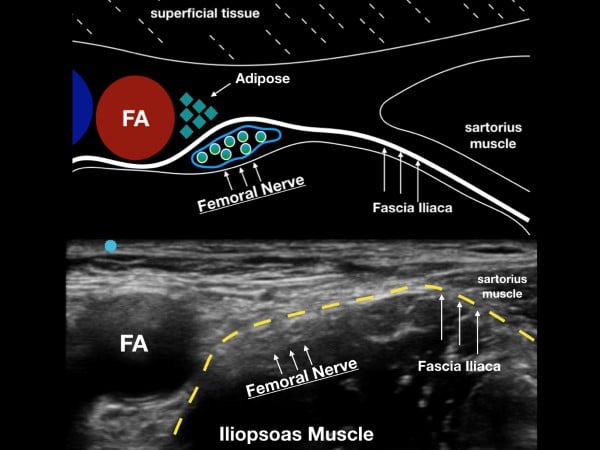
The femoral nerve sits laterally to the femoral artery and vein. The fascia iliaca, which holds the femoral nerve tightly to the underlying iliopsoas muscle, is the target when performing the UGFNB. The goal of the block is to gently inject LA under this fascial plane and bathe the femoral nerve in local anesthetic (LA). (fig 2)
Figure 2. The femoral nerve sits lateral to the femoral artery (FA) and under the fascia iliaca. Note the fascia iliaca holds the femoral nerve close to the iliopsoas muscle.
Survey Scan
With the patient in a supine position, place the high-frequency linear transducer just below and parallel to the inguinal crease. Locate the femoral artery superior to its bifurcation and vein in cross-section, and then note the femoral nerve just lateral to the vascular bundle and below the fascia iliaca. The femoral nerve bundle will appear as a hyperechoic, triangular structure with a "honeycomb" appearance. The femoral nerve commonly lies at the inferior aspect of the femoral bundle and may not be easily seen on the initial ultrasound survey. The clinician should note the fascia iliaca that runs laterally from the femoral nerve bundle and over the iliopsoas muscle. In cases where the fascia iliaca is challenging to identify, slide the transducer laterally until the sartorius muscle is noted. The hyperechoic fascia iliaca will run below the sartorius muscle but above the iliopsoas muscle. The goal of the block is to place anesthetic under the fascia iliaca about 1-3 cm lateral to the femoral nerve complex.
Technique
Once the area is prepped with chlorhexidine (or similar skin cleanser), place a 1-2 mL skin wheal of anesthetic lateral to the probe. Using an in-plane lateral to medial approach, advance the needle tip under the fascia iliaca (lateral to medial), and gently inject 1-2 mL of normal saline. Anechoic fluid should be visible spreading under the fascia iliaca. The anesthetic solution can then be gently injected in 3-5 mL aliquots (aspirating in between each dose to ensure a lack of inadvertent vascular puncture) until the desired volume is reached. The anechoic anesthetic should track under the fascia iliaca and toward the femoral nerve bundle. Often, fluid will separate the superficial adipose tissue from the femoral nerve that is adjacent to the iliopsoas muscle. (fig 3)
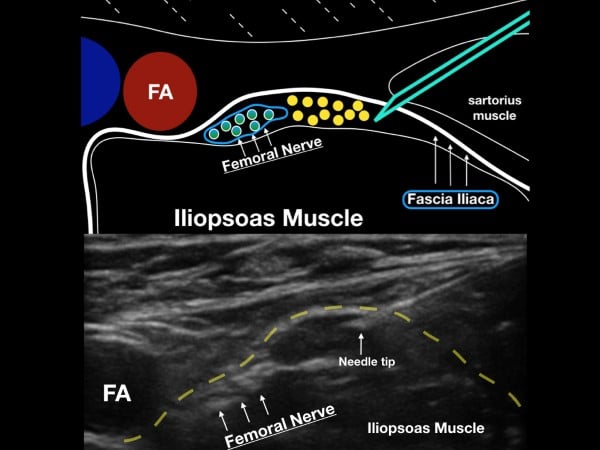
Figure 3. The goal of the ultrasound-guided femoral nerve block will be to get anesthetic under the fascia iliaca (a few centimeters laters to the femoral nerve).
Serratus Anterior Plane Block
Indications
The ultrasound-guided serratus anterior plane block (SAPB) provides anesthesia to the lateral and anterior hemithorax. In the emergency department, this mid-axillary plane block is ideal for pain control for acute rib fractures, burns, chest wall abscesses, and thoracic zoster. By utilizing a large volume of dilute anesthetic, a single injection tracks up and down within the fascial plane and can provide anesthesia over multiple vertebral levels.1 The posterior thorax is primarily innervated from branches of the dorsal rami, which are not reached by the SAPB. Therefore, the SAPB does not reliably provide pain control for posterior rib fractures, nor does it consistently anesthetize the pleura during the insertion of thoracostomy tubes.2
Anatomy
The intercostal nerves derive from the anterior rami of the thoracic spinal nerves and travel laterally along the hemithorax within the intercostal muscles. In the mid-axillary line, the lateral cutaneous branches pierce the internal and external intercostal muscles to innervate the muscles and skin of the lateral and anterior hemithorax. The SAPB deposits local anesthetic into the fascial plane overlying the anterior surface of the serratus anterior muscle directly targeting the lateral cutaneous branches. Pain relief for rib fractures is thought to occur through the spread of local anesthetic along these penetrating branches deep to the intercostal nerves.5-6 (fig 4)
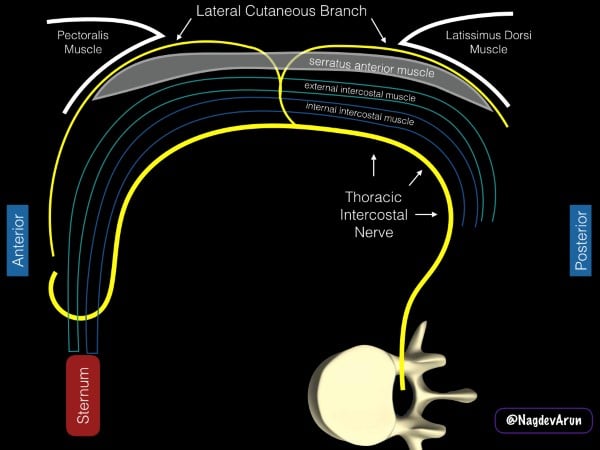
Figure 4. Schematic representation of the anatomy of the serratus anterior plane block. The drawing is based on a patient being placed in a lateral decubitus position with the pectoralis muscle anterior and the latissimus dorsi muscle posterior.
Positioning
The SAPB can be performed with the patient either in the lateral decubitus position or supine (if unable to roll due to concurrent cervical, spinal, or extremity injuries). When performing the block with the patient in the lateral decubitus position (affected side up), the provider should be behind the patient with the ultrasound system contralateral and in direct line of sight. The needle trajectory will be posterior to anterior on the patient (fig 5). In the supine position, the provider stands on the ipsilateral side of the injury near the patient's head with the ultrasound system caudal. With this positioning, the needle will travel anterior to posterior on the patient.
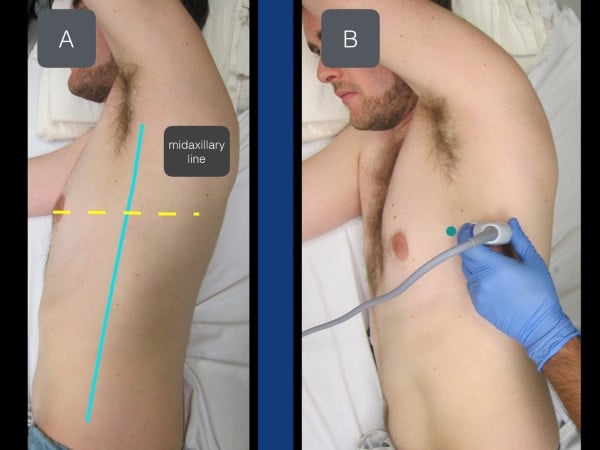
Figure 5. With the patient in a lateral decubitus position (affected side up), the transducer is placed in a transverse plane at the level of the nipple (in between the pectoralis muscle and latissimus dorsi muscle).
Survey Scan
The thoracic level of the block is to be targeted at the midpoint of the identified fractures. For example, if a patient has fractures of T 3,4,5 ribs, the level of injection will be at T4. If the patient has fractures at T6-10, the level of injection will be at T8. The nipple line and inferior border of the scapula are approximately T4 and T5. Place the linear transducer in transverse orientation at the selected level at the mid-axillary line so that the ribs are visualized in the cross-section. The muscle layer superficial to the rib (and resultant shadow) is the serratus anterior muscle. If the transducer is placed in the posterior axillary line, superficial to the serratus anterior is the tapering lateral border of the latissimus dorsi muscle. Above the muscles is a layer of subcutaneous tissue, and deep to the ribs is the hyperechoic pleural line (fig 6).
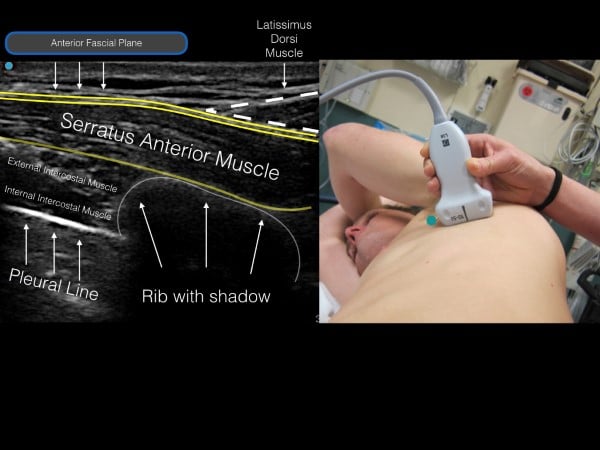
Figure 6. Ultrasound image (with corresponding transducer placement) of the serratus muscle, rib shadow and pleural line. Note the anterior fascial plane above the serratus muscle (location for anesthetic deposition).
Technique
The SAPB will typically use 30 mL of long-acting local anesthetic depending on the number of vertebral levels intended to cover. Always refer to a weight-based dosing guide (highlandultrasound.com/med-guide) to minimize the risk of local anesthetic systemic toxicity. This large volume block is more easily performed by a two-person technique using a 20-22-gauge needle (3.5-inch spinal for large patients) connected by extension tubing to a 30 mL syringe.
Cleanse the skin with chlorhexidine and make a skin wheal using 1-2 cc of anesthetic. Using an in-plane technique, insert the needle through the anesthetized skin wheal and advance under real-time ultrasound guidance to the intermuscular plane between the latissimus dorsi and the anterior surface of the serratus anterior muscle. The assistant will then aspirate and gently inject a test dose of 1-2 cc of normal saline. If the needle is positioned within the inter-muscular fascial plane, anechoic fluid will spread laterally and open up this potential space. Conversely, if the fluid travels retrograde up the needle shaft or within muscle bellies of the serratus anterior or latissimus dorsi, reposition the needle tip into the fascial plane and perform another small volume test injection until there is a clear separation of the two muscles. Intramuscular injections are the most common cause of block failure. Once in the correct intrafascial plane, aspirate again and begin slow injections in aliquots of 3-5 cc until the full volume of local anesthetic has been deposited into the fascial plane (fig 7).
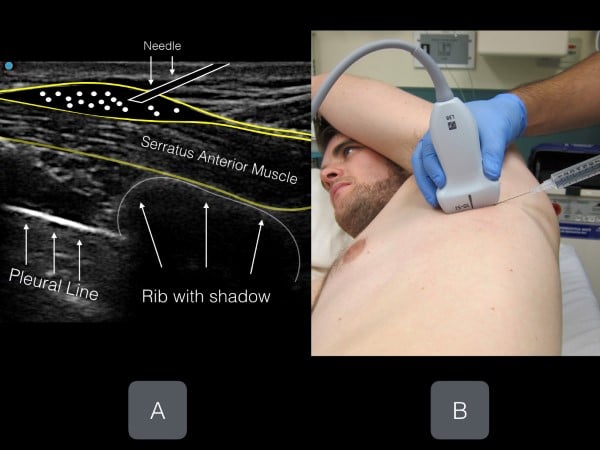
Figure 7. Clear needle tip visualization of anesthetic being injected in the fascial plane above the serratus anterior muscle. The goal is for anesthetic fluid to open the potential space and track anteriorly or posteriorly (or in both directions)
Interscalene Nerve Block
Indications
Blockade of the interscalene brachial plexus can be used to provide anesthesia to the shoulder and proximal upper extremity. There are a variety of indications for interscalene brachial plexus block, including analgesia for proximal and midshaft humeral fractures, upper extremity abscess drainage, wound debridement, and complex laceration repair. The interscalene brachial plexus block can also be utilized as an alternative to procedural sedation for the reduction of glenohumeral dislocations. While the interscalene brachial plexus block provides anesthesia to the upper arm, it usually does not reliably extend beyond the elbow.7-9
Contraindications and complications
High volume interscalene brachial plexus blocks (> 20-30 mL) are commonly known to cause ipsilateral hemidiaphragm paralysis because of the inadvertent spread of anesthetic over the anterior scalene muscle and onto the phrenic nerve. The clinical significance of temporary hemidiaphragm paralysis in healthy patients is debated; however, caution is advised in patients with compromised respiratory function. In patients with known respiratory compromise, we recommend either a low volume block (< 10 mL) or not performing the procedure.
Anatomy
The C5-C7 nerve roots can be targeted as they pass between the anterior and middle scalene muscles, typically lying far lateral to the carotid artery (pic 8 and 9). The superior thyroidal artery and its branches lie medially and superiorly, while the cervical arteries are usually posterior and lateral. The dome of the pleura lies inferiorly and can easily be avoided with proper needle tip visualization. Anatomic variability of small vascular structures in the vicinity of the interscalene groove warrants consideration for patient selection and safety. We recommend using color Doppler (if possible) to inspect the needle path whenever performing this block because of the variant anatomy on the neck.
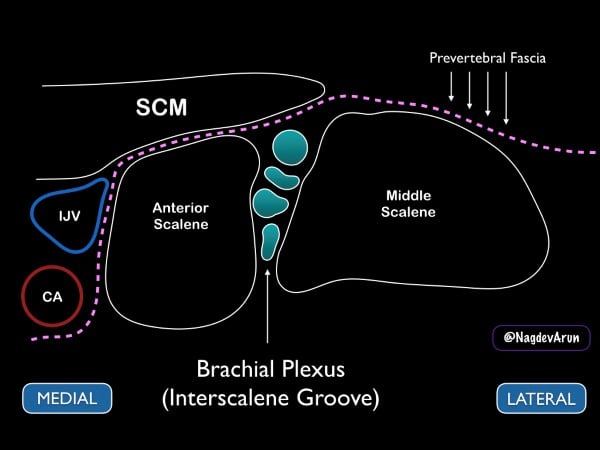
Figure 8. Drawing of the brachial plexus at the interscalene groove. Note the medial sternocleidomastoid muscle (SCM), internal jugular vein (IJV) and carotid artery (CA).
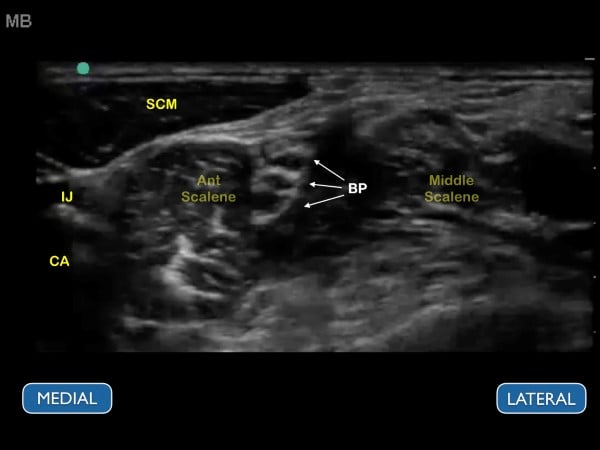
Figure 9. Ultrasound image of the brachial plexus at the interscalene groove. The ultrasound image should correspond to the previous figure
Survey Scan
Ideally, the patient should be placed supine with the head of the bed elevated to approximately 30 degrees. A towel or "bump" can be placed under the affected shoulder to rotate the thorax so that the needle can be advanced in at a flat angle (this allows for better needle visualization). With the patient’s head turned away from the affected side, place a high-frequency linear transducer on the neck lateral to the cricoid cartilage. For novice sonographers who are unfamiliar with the regional anatomy of the brachial plexus, we recommend first to identify the carotid artery and internal jugular vein. Slide the transducer lateral until the sternocleidomastoid muscle can be seen lying superficially to the anterior scalene muscle. By sliding the transducer just laterally, the interscalene groove can be identified between the anterior and middle scalene by the characteristic traffic light appearance of the C5-C7 nerve roots lying vertically between the muscle bodies. If possible, we recommend placing color Doppler over the hypoechoic circular nerve roots to ensure that they are not vasculature.
The brachial plexus at the interscalene groove can also be identified by initially locating the brachial plexus in the supraclavicular fossa. Place the high-frequency linear transducer parallel to the clavicle and identify the subclavian artery (in cross-section) and the adjacent brachial plexus just lateral. The brachial plexus at this location will be lateral to the subclavian artery and have its characteristic honeycomb appearance. From this location, slowly slide the probe up the neck, tracing the bundle up the neck and to the classic interscalene location. (fig 10)
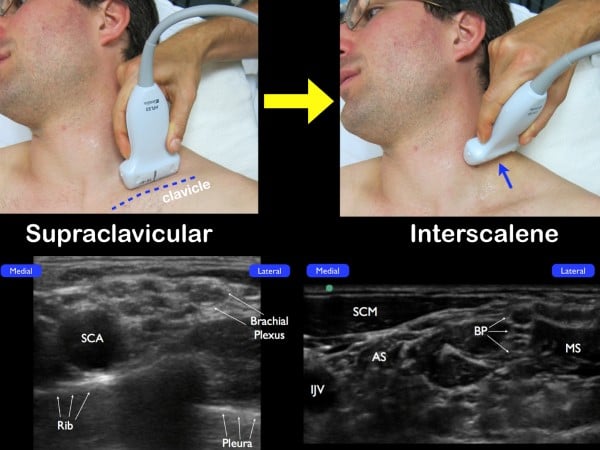
Figure 10. If you are unable to easily identify the brachial plexus at the interscalene groove, place the transducer parallel to the clavicle and fan anteriorly in a coronal plane. Once the subclavian artery (SCA) and the brachial plexus (just lateral) are noted, slide the transducer up the neck in a transverse plane (while visualizing the nerve bundle). The anterior scalene muscle (AS) and middle scalene muscle (MS) will be seen with the interscalene brachial plexus in the center.
Technique
After identifying the C5-C7 nerve roots and other relevant anatomical structures, a small skin wheal should be placed approximately 1 cm lateral to the probe. Using the skin wheel as the point of entry, a 21 to 23 gauge 1 ½ inch block needle should be advanced under ultrasound guidance using an in-plane technique. The needle tip is then advanced just above the middle scalene muscle. The goal is to place anesthetic just under the prevertebral fascia that overlays the superficial border of the middle scalene muscle. Ideally, the anechoic fluid will spread down the interscalene groove in between the brachial plexus and the middle scalene muscle, but this may not always be possible. When the needle tip is appropriately positioned, a local anesthetic will spread along the fascial plane, separating the nerve roots from the middle scalene. (fig 11 and 12) Often, small needle tip adjustments are needed to achieve anesthetic spread along the fascial plane. While it is desirable to have proximity between the needle tip and nerve sheath, an inter-neuronal injection should be avoided. The block should be stopped, and the needle tip adjusted if pain or resistance with the injection of local anesthetic is encountered. The sonographer should be aware of these early signs of intraneural injections. Care should be taken to maintain visualization of the needle tip and spread of local anesthetic throughout the procedure. If needle tip visualization cannot be achieved or local anesthesia is not seen spreading, the procedure should be stopped.
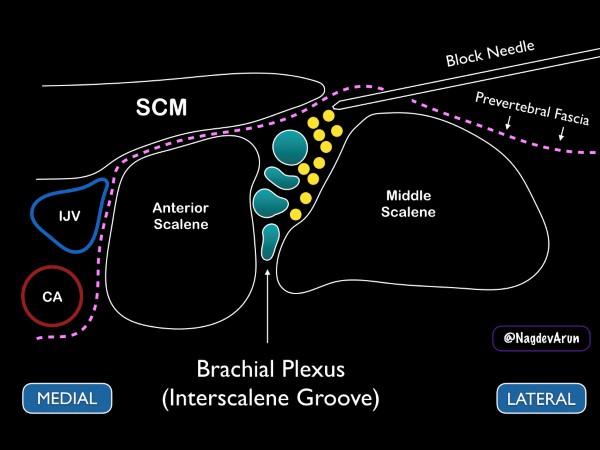
Figure 11. Drawing demonstrating how to place anesthetic in the interscalene groove
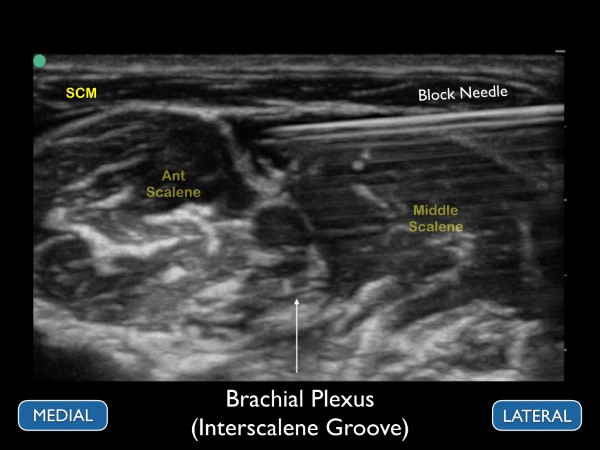
Figure 12. Ultrasound image with anechoic anesthetic placed under the prevertebral fascia and adjacent to the interscalene brachial plexus
Summary
Following consideration of both the safety and efficacy measures, nerve blocks serve as an excellent adjunct to pain presentations in the emergency setting. A stepwise approach to pre-block assessment, anesthetic administration, and post-block reassessment allow emergency providers to minimize pain while also limiting adverse effects associated with anesthetic injection.
References
- Gottlieb M, Chien N, Seagraves T. How effective is a regional nerve block for treating pain associated with hip fractures? Ann Emerg Med. 2018;71(3):378-380.
- Dickman E, Pushkar I, Likourezos A, et al. Ultrasound-guided nerve blocks for intracapsular and extracapsular hip fractures. Am J Emerg Med. 2016;34(3):586-589.
- Beaudoin FL, Haran JP, Liebmann O. A comparison of ultrasound-guided three-in-one femoral nerve block versus parenteral opioids alone for analgesia in emergency department patients with hip fractures: a randomized controlled trial. Acad Emerg Med. 2013;20(6):584-591.
- Ketelaars R, Stollman JT, van Eeten E, Eikendal T, Bruhn J, van Geffen G-J. Emergency physician-performed ultrasound-guided nerve blocks in proximal femoral fractures provide safe and effective pain relief: a prospective observational study in The Netherlands. Int J Emerg Med. 2018;11(1):12.
- Blanco R, Parras T, McDonnell J, Galino A. Serratus plane block: a novel ultrasound-guided thoracic wall nerve block. Anesthesia. 2013; 68:1107-13.
- Mayes J, Davison E, Panahi P et al. An anatomical evaluation of the serrates anterior plane block. Anesthesia. 2016;71(9):1064-9.
- Mcgahan J, Nagdev A, McCarthy C, Martin, D, Nerve Blocks,. Fundamentals of Emergency Ultrasound, 2019; 362-367.
- Hussain N, Goldar G, Ragina N, Banfield L, Laffey JG, Abdallah FW. Suprascapular and interscalene nerve block for shoulder surgery: a systematic review and meta-analysis. Anesthesiology. 2017;127(6):998–1013.
- Melton MS, Monroe HE, Qi W, Lewis SL, Nielsen KC, Klein SM. Effect of interscalene brachial plexus block on the pulmonary function of obese patients: a prospective, observational cohort study. Anesth Analg. 2017;125(1):313–319.