Ch. 6 - Back Pain
David H. Cisewski, MD, MS | Icahn School of Medicine at Mount Sinai
Benjamin W. Friedman, MD, MS | Albert Einstein College of Medicine
Back pain is estimated to affect 1 in 5 people worldwide,1 leading to more global disability than any other medical condition.2,3 In the United States alone, back pain results in an estimated 2.6 million emergency department (ED) visits annually.4
Additionally, back pain constitutes $600 billion in direct and indirect costs annually in the U.S.,5 with an estimated 50% of all patients reporting functional impairment in activity of daily living secondary to pain in the week following ED visit.6 If allowed to become chronic, back pain may result in depression, work-related stress, and exacerbation of other underlying medical pathologies.7-9
Despite the prevalence of back pain and the effect on society, we have yet to identify the "magic bullet." Approximately 70% of all patients treated for back pain in the emergency department continue to have pain a week following ED presentation while up to one-third continue to have pain at three months.10 The good news is that most patients with acute or subacute back pain will improve over time regardless of treatment modality. As such, clinicians should focus on a combination of both pharmacologic and non-pharmacologic treatment modalities to address the pain.11 The challenge remains how to treat these pain presentations in the acute setting.
BACK PAIN DIFFERENTIAL DIAGNOSIS
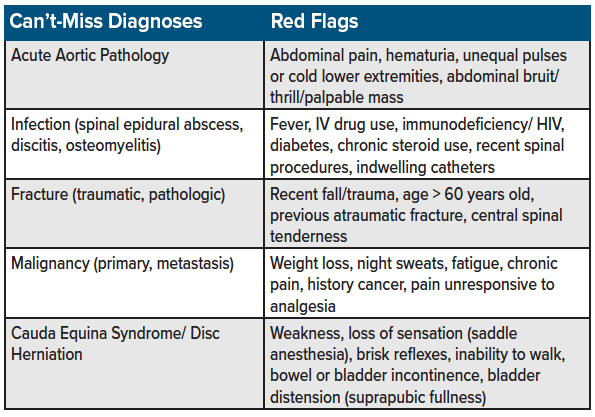
As with all pain presentations, the initial consideration should focus on ruling out the life-threatening causes (see table for a list of “can't-miss” diagnoses for back pain presentations). This chapter assumes the treatment of acute back pain in which serious pathologies have been considered and ruled out. (Note: The treatment of alternative sources of pain such as chronic pain, cancer pain, and fracture-related pain, will be covered in separate chapters.) See table 1 for a list of can’t-miss diagnoses on the differential for back pain in the emergency setting.
TREATING BACK PAIN
In 2007, the American Pain Society (APS) and the American College of Physicians (ACP) released joint guidelines for the treatment of back pain, with non-steroidal anti-inflammatory drugs (NSAIDs) being listed as first-line analgesics while acetaminophen, opioids, benzodiazepines, and skeletal muscle relaxants (SMR) were listed as second-line treatment alternatives.12 This recommendation was not based on the superiority of NSAIDs for treating back pain, but rather the decreased risk of adverse effects compared to the alternatives. Despite the risk of these adverse effects, a retrospective review has shown that opioids (63% of visits), SMRs (43%), and benzodiazepines (11%) are used extensively in the treatment of acute atraumatic back pain.4 In 2017, the ACP updated their recommendations in favor of a combination of both pharmacologic and non-pharmacologic modalities.11 This chapter will focus on the evidence behind these recommendations for acute pain management in the inpatient setting, as well as a brief discussion on the non-pharmacologic modalities that can be utilized in the outpatient setting.
PHARMACOLOGIC TREATMENTS
Non-steroidal anti-inflammatory drugs (NSAIDs)
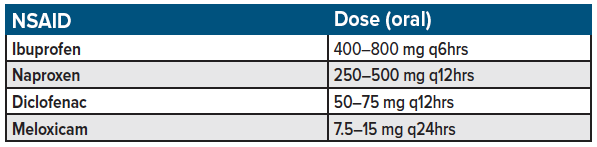
NSAIDs such as ibuprofen, diclofenac, naproxen, ketorolac remain the first-line standard for acute, non-radicular back pain treatment in the emergency setting (see table 2).12
Despite NSAIDs being the gold standard, up to one-third of patients who receive NSAIDs alone for ED presentations of back pain report moderate or severe low back pain at a one-week follow-up.10 A recent systematic review and meta-analysis found NSAIDs resulted in a small but clinically significant improvement in back pain with a number-needed-to-treat (NNT) of 6 (95% CI 4 to 10) for one additional participant to achieve clinically important pain reduction over placebo.13 NSAIDs were also shown to increase the risk of gastrointestinal adverse events by 2.5 times (95% CI 1.2 to 5.2). The authors concluded that NSAIDs were effective for back pain, but that the magnitude of the pain reduction over placebo had little clinically important effect while increasing the susceptibility to adverse effects indicating a need for alternative treatment modalities.
Bottom line: Consider a trial of NSAIDs as a first-line treatment of back in the acute setting; among patients responsive to NSAID analgesia in the ED, offer an additional short-course of NSAIDs (3-5 days) to limit adverse effects of continued use.
Acetaminophen
Acetaminophen was previously listed as a first-line regimen in the 2007 joint ACP/APS recommendations for acute back pain.12 However, a meta-analysis assessing acetaminophen for low back pain found that acetaminophen (paracetamol) resulted in no difference in pain reduction between paracetamol (4 g per day) and placebo at 1 week, 2 weeks, 4 weeks, and 12 weeks for back pain reduction.14 Additionally, paracetamol did not affect quality of life, function, global impression of recovery, and sleep quality for all included periods. Despite their inclusion as a treatment regimen in the 2007 ACP/APS joint recommendations, the updated 2017 recommendations indicate that acetaminophen, either as mono-therapy or combined with NSAIDs, is not effective for LBP.11
Bottom line: Acetaminophen is no longer recommended in the treatment of acute back pain.
Opioids
The use of opioids for back pain is based on the known efficacy of opioids for alternative pain pathologies.15,16 In the emergency setting, opioid combination analgesics (acetaminophen-codeine) were shown to be no more effective than NSAIDs (ketorolac) alone for acute (short-term) pain relief. However, the opioid combinations were associated with increased adverse effects.17 These results were consistent with research showing opioid combination analgesics (acetaminophen-oxycodone) were no more effective than NSAIDs (valdecoxib) alone for acute musculoskeletal pain relief, again with the opioid combinations being associated with increased adverse effects.18 Additionally, a randomized control trial comparing the use of opioids (oxycodone/acetaminophen) as an adjunct to NSAIDs (naproxen) for acute, non-radicular, low back pain showed opioids were no more effective in improving functional outcomes or extended pain reduction at 1-week follow-up compared to NSAIDs alone.19 Research has also shown a negative association between early opioid use for acute back pain and outcomes such as long-term disability and opioid use, suggesting the use of opioids may be counterproductive to recovery.20 Additionally, when controlling for pain acuity, injury pattern, and functional severity, research has shown that those patients who received greater than a one-week prescription for opioids were twice as likely to have continued work-related disability at one-year follow-up.21
Bottom line: due to transient analgesic relief, no evidence of improvement in functional outcome or improvement in long-term disability opioids are not recommended as a first-line analgesic regimen for acute back pain. Opioids should be reserved for patients in which other alternatives have been exhausted and in which a low-dose regimen in the emergency setting would allow a return to mobilization.
Skeletal Muscle Relaxants (SMR)
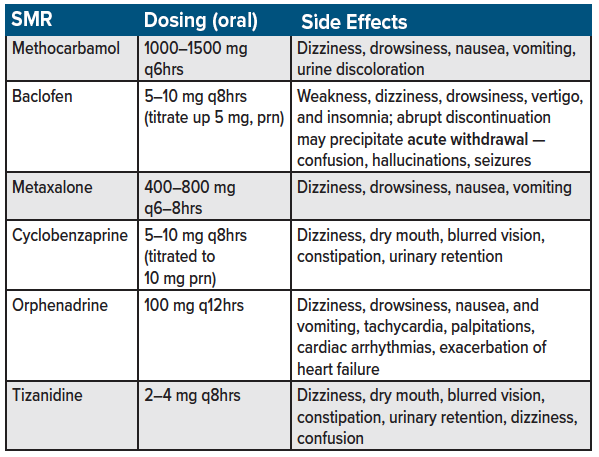
Skeletal muscle relaxants (SMRs) are a second-line agent for acute back pain management.11,12 Examples of SMRs include methocarbamol, cyclobenzaprine, orphenadrine, carisoprodol, tizanidine, metaxalone, and baclofen (see table 3 for a list of commonly used skeletal muscle relaxants used for acute back pain in the emergency department). Estimates suggest up to 35% of patients with nonspecific low back pain are prescribed SMRs,22,23 with orphenadrine, and methocarbamol being used in more than 250,000 U.S. ED visits for low back pain annually.24 Despite their branding as muscle relaxants, the anti-spasmodic and analgesic effects of SMRs are predominantly due to unknown mechanism of action.22
Several SMRs have been shown to be superior to placebo in short-term pain reduction associated with acute, non-radicular back pain.25,26 However, research conducted in the emergency setting has demonstrated the addition of SMRs to NSAIDs were neither superior to NSAIDs alone in improving overall function nor in reducing acute, non-radicular low back pain.6,24
Concerns have been raised regarding the adverse effects of SMRs as a treatment modality in back pain.27 Side effects such as dizziness, headaches, blurred vision, nausea and vomiting, dry mouth, urinary retention, constipation, confusion, risk of falls or fractures, and dependency may have an adverse effect on patient recovery and quality of life.22 Additionally, some SMRs have a high abuse potential and risk of diversion secondary to their sedating effects when used concomitantly with benzodiazepines or alcohol, and their ability to reduce jitteriness during cocaine consumption.28 SMRs are also listed among the Beers Criteria of medications to avoid among geriatric patients.29 Research has shown that among patients 65 years and older with an injury resulting in hospitalization, 1.3% cases (vs. 0.8% controls) were found to be concomitantly prescribed SMRs (adjusted OR = 1.32), highlighting the importance of limiting SMRs among the geriatric population.30
SMRs are often taken in higher quantities and for longer durations than recommended in the treatment of low back pain.22 Given the severity of adverse effects, extended prescriptions pose a threat to patient recovery in the outpatient setting.
Bottom line: Due to lack of efficacy over NSAIDs alone as well as high risk of adverse effects, SMRs for acute back pain should be limited to patients who cannot tolerate NSAIDs or who have not reported sufficient relief with NSAIDs. If provider discretion indicates that the benefits outweigh the risks, a short 2- to 3-day prescription with a full patient discussion regarding patient anticipated side effects should be considered.
Gabapentinoids (pregabalin, gabapentin)
Anticonvulsants such as pregabalin and gabapentin have been considered an adjunct for patients with radiating or neuropathic back pain.31 However, these medications do not improve pain or disability secondary to lower back pain with or without a radiating component.31,32 Side effects of both gabapentin and pregabalin include dizziness, fatigue and drowsiness, ataxia, peripheral edema, nystagmus, and tremor, with drowsiness and dizziness being the most commonly cited among studies and a major cause of study drop-out.33,34 Caution should be taken in elderly patients, in which gabapentin can cause or exacerbate cognitive impairments.34 Gabapentinoids have an additional increased risk of diversion and potential for abuse secondary to their ability to potentiate the effects of opioids during concomitant use.35
Bottom line: Gabapentinoids are not recommended for the routine treatment of acute (or chronic) back pain in the emergency setting.
Benzodiazepines
Similar to muscle relaxants, benzodiazepines are often added to NSAIDs as an analgesic adjunct secondary to their relaxing (anxiolytic) effect. Despite a lack of evidence, benzodiazepines are used extensively for ED-related back pain presentations.4 However, research in the ED-setting has shown the use of benzodiazepines (diazepam) as an NSAID adjunct offers no improvement in functional outcomes or pain at one week and three months follow-up compared to NSAIDs alone.36 Use of benzodiazepines for acute back pain must further be weighed against the adverse effects which include drowsiness, dizziness, fall risk, slurred speech, nausea, confusion, abuse, and dependence.37,38 Similar to SMRs, benzodiazepines are also listed among the Beers Criteria of medications to avoid among the geriatric population.29
Bottom line: Despite the anxiolytic and relaxing effects of benzodiazepines, benzodiazepines are not recommended as a first-line regimen in the treatment of acute back pain in the emergency setting.
Topical Analgesics
As opposed to oral and intravenous analgesics that require systemic distribution to obtain the effect, topical/transdermal analgesics are a localized form of analgesic which limits total systemic quantities delivered.39,40 Topical analgesics are optimal in patients with low back pain complicated by renal disease, elderly patients susceptible to elevated analgesic plasma concentrations, or in patients with multiple comorbidities such as peptic ulcer disease and cardiovascular disease in which oral NSAIDs are relatively contraindicated.41 Examples include diclofenac, ketoprofen, ibuprofen, 5% lidocaine patch, EMLA cream, and capsaicin cream, methyl salicylate.
A 2017 Cochrane review showed gel formulations such as diclofenac, ibuprofen, and ketoprofen provided improved analgesia among those with acute musculoskeletal pain.42 These results were consistent with the 2010 Cochrane review, which found a number needed to treat (NNT) for clinical success (equivalent to 50% pain relief) of topical NSAIDs (diclofenac, ibuprofen, ketoprofen, and piroxicam) of 4.5 for treatment periods of 1-2 weeks.43 Though data is limited, similar analgesic relief has been demonstrated among patients with low back pain.44
Bottom line: Topical NSAIDs may be considered a safe alternative among patients with acute back pain in which oral NSAIDs are contraindicated.
Trigger Point Injections
A trigger point is a focal, palpable area of tenderness that is reproducible with palpitation.45 In the emergency setting, a local anesthetic injection – termed trigger point injection (TPI) - may offer temporary relief of acute back pain secondary to myofascial irritation.46 Commonly used anesthetics include lidocaine 2% or bupivacaine 0.25-0.5%. Similar to topical analgesics, TPIs offer a focal, non-distributive form of analgesic relief with limited adverse effects. The following materials are needed to perform a TPI:
- Needle (1.5–2.5 in 25 or 27- gauge) and syringe (5–10cc)
- Local anesthetic (3–5 cc bupivacaine 0.25-0.5%)
- Sterilization - alcohol swab, chlorhexidine wipe
To perform a TPI, the first step is to identify the trigger point by palpating along the lower lumbar region until the point of maximum tenderness is noted by the patient. Following identification and sterilization with an isopropyl alcohol swab or chlorhexidine wipe, immobilize the skin surround the trigger point with the non-dominant hand. Using the dominant hand, insert the needle approximately 1-3 cm below the skin surface. A “twitch” sensation may be noted, indicating the site of myofascial band tension. Following negative aspiration, inject 3-5 cc local anesthetic in a fanning distribution surrounding the site of tenderness. A brief post-injection tissue massage may assist in anesthetic distribution.
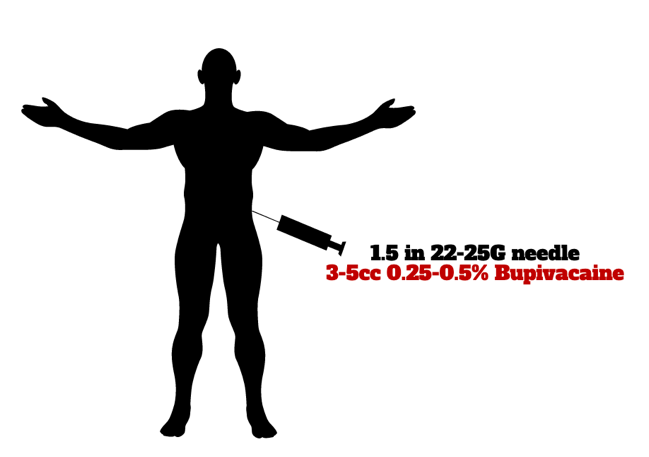
Image 1: Trigger point injection (TPI)
Despite an argument for “dry needling” (needle injection without anesthesia), the anesthetic injection has been shown to results in reduced post-injection soreness and longer duration of analgesic effect.47
Bottom line: Consider a trigger point injection as an analgesic adjunct in patients with focal, palpable areas of tense bands of tenderness that is reproducible with palpitation. Despite a relatively high safety profile of this localized form of analgesic relief, further research is needed to determine the efficacy.
Non-pharmacologic Treatment Modalities
Due to the growing body of evidence that most pharmacologic therapies do little to improve acute back pain or long-term functionality, additional efforts have been made to identify and promote non-pharmacologic treatment alternatives.11 The 2017 ACP clinical practice guidelines have listed several non-pharmacologic modalities that may be considered in patients experiencing low back pain (see table 4 for a list of non-pharmacologic treatment modalities used for acute back pain in the outpatient setting).
Despite a limited amount of evidence, few differences were seen in terms of long-term pain relief or functional outcome between the various non-pharmacologic modalities. The only widely accepted recommendation was to avoid bed rest and remain active. Unfortunately, many of the non-pharmacologic interventions had weak evidence to support their efficacy and significant patient noncompliance in the outpatient setting.24 Additionally, many of the non-pharmacologic treatment modalities are limited by patient accessibility and cost (lack of insurance reimbursement).48,49 Nevertheless, given the low risk of adverse effects and potential for general health and wellness benefit, these modalities should be discussed as outpatient alternatives with all patients presenting with acute back pain.
Bottom line: Non-pharmacologic treatment alternatives such as yoga, ice/heat, exercise, tai chi, acupuncture, nerve stimulation, and minimization of bed rest should be recommended to all patients presenting with acute back pain.
Summary
As demonstrated throughout this review of acute back pain treatment, no "magic bullet" exists to treat these pain presentations in the ED setting. NSAIDs remain the gold standard treatment recommendation, though, though only offer a small but clinically significant improvement in pain. In the acute setting, a trial of topical analgesics or a trigger point injection are considered safe analgesic alternatives though research is needed to support their use. Skeletal muscle relaxants may be considered in the acute setting when NSAIDs are not effective or contraindicated, though side effect profile should be weighed against the benefit of treatment. Opioids carry an increased risk of abuse and adverse effects and should be limited among patients with acute back pain. Gabapentinoids show no efficacy in comparison to placebo. It is essential to set patient expectations so that they are aware of the slow but steady improvement and that remaining active (without bedrest) remains the most universally-accepted treatment recommendation. Providing a list of non-pharmacologic alternatives may assist with outpatient recovery and functional improvement.
References
- Hoy D, Bain C, Williams G, et al. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012;64:2028-2037.
- Hoy D, March L, Brooks P, et al. The global burden of low back pain: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73:968-974.
- Global Burden of Disease Cancer C, Fitzmaurice C, Allen C, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524-548.
- Friedman BW, Chilstrom M, Bijur PE, Gallagher EJ. Diagnostic testing and treatment of low back pain in United States emergency departments: a national perspective. Spine (Phila Pa 1976). 2010;35:E1406-11.
- Deyo RA, Dworkin SF, Amtmann D, et al. Report of the NIH Task Force on research standards for chronic low back pain. Phys Ther. 2015;95:e1-e18.
- Friedman BW, Irizarry E, Solorzano C, et al. A Randomized, Placebo-Controlled Trial of Ibuprofen Plus Metaxalone, Tizanidine, or Baclofen for Acute Low Back Pain. Ann Emerg Med. 2019;74(4):512-520.
- Wertli MM, Burgstaller JM, Weiser S, Steurer J, Kofmehl R, Held U. Influence of catastrophizing on treatment outcome in patients with nonspecific low back pain: a systematic review. Spine (Phila Pa 1976). 2014;39:263-273.
- Pinheiro MB, Ferreira ML, Refshauge K, et al. Symptoms of depression as a prognostic factor for low back pain: a systematic review. Spine J. 2016;16:105-116.
- Henschke N, Maher CG, Refshauge KM, et al. Prognosis in patients with recent onset low back pain in Australian primary care: inception cohort study. BMJ. 2008;337:a171.
- Friedman BW, Conway J, Campbell C, Bijur PE, John Gallagher E. Pain One Week After an Emergency Department Visit for Acute Low Back Pain Is Associated With Poor Three-month Outcomes. Acad Emerg Med. 2018;25:1138-1145.
- Qaseem A, Wilt TJ, McLean RM, Forciea MA, Clinical Guidelines Committee of the American College of P. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med. 2017;166:514-530.
- Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147:478-491.
- Machado GC, Maher CG, Ferreira PH, Day RO, Pinheiro MB, Ferreira ML. Non-steroidal anti-inflammatory drugs for spinal pain: a systematic review and meta-analysis. Ann Rheum Dis. 2017;76:1269-1278.
- Saragiotto BT, Machado GC, Ferreira ML, Pinheiro MB, Abdel Shaheed C, Maher CG. Paracetamol for low back pain. Cochrane Database Syst Rev. 2016:CD012230.
- Deyo RA, Von Korff M, Duhrkoop D. Opioids for low back pain. BMJ. 2015;350:g6380.
- Chou R, Huffman LH, American Pain S, American College of P. Medications for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:505-514.
- Innes GD, Croskerry P, Worthington J, Beveridge R, Jones D. Ketorolac versus acetaminophen-codeine in the emergency department treatment of acute low back pain. J Emerg Med. 1998;16:549-556.
- Lovell SJ, Taira T, Rodriguez E, Wackett A, Gulla J, Singer AJ. Comparison of valdecoxib and an oxycodone-acetaminophen combination for acute musculoskeletal pain in the emergency department: a randomized controlled trial. Acad Emerg Med. 2004;11:1278-1282.
- Friedman BW, Dym AA, Davitt M, et al. Naproxen With Cyclobenzaprine, Oxycodone/Acetaminophen, or Placebo for Treating Acute Low Back Pain: A Randomized Clinical Trial. JAMA. 2015;314:1572-1580.
- Webster BS, Verma SK, Gatchel RJ. Relationship between early opioid prescribing for acute occupational low back pain and disability duration, medical costs, subsequent surgery and late opioid use. Spine (Phila Pa 1976). 2007;32:2127-2132.
- Franklin GM, Stover BD, Turner JA, Fulton-Kehoe D, Wickizer TM, Disability Risk Identification Study C. Early opioid prescription and subsequent disability among workers with back injuries: the Disability Risk Identification Study Cohort. Spine (Phila Pa 1976). 2008;33:199-204.
- Witenko C, Moorman-Li R, Motycka C, et al. Considerations for the appropriate use of skeletal muscle relaxants for the management of acute low back pain. P T. 2014;39:427-435.
- Cherkin DC, Wheeler KJ, Barlow W, Deyo RA. Medication use for low back pain in primary care. Spine (Phila Pa 1976). 1998;23:607-614.
- Friedman BW, Cisewski D, Irizarry E, et al. A Randomized, Double-Blind, Placebo-Controlled Trial of Naproxen With or Without Orphenadrine or Methocarbamol for Acute Low Back Pain. Ann Emerg Med. 2018;71:348-56 e5.
- Tisdale SA, Jr., Ervin DK. A controlled study of methocarbamol (Robaxin) in acute painful musculoskeletal conditions. Curr Ther Res Clin Exp. 1975;17:525-530.
- Emrich OM, Milachowski KA, Strohmeier M. [Methocarbamol in acute low back pain. A randomized double-blind controlled study]. MMW Fortschr Med. 2015;157 Suppl 5:9-16.
- van Tulder MW, Touray T, Furlan AD, Solway S, Bouter LM, Cochrane Back Review G. Muscle relaxants for nonspecific low back pain: a systematic review within the framework of the cochrane collaboration. Spine (Phila Pa 1976). 2003;28:1978-1992.
- Reeves RR, Burke RS, Kose S. Carisoprodol: update on abuse potential and legal status. South Med J. 2012;105:619-623.
- American Geriatrics Society Beers Criteria Update Expert P. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616-631.
- Spence MM, Shin PJ, Lee EA, Gibbs NE. Risk of injury associated with skeletal muscle relaxant use in older adults. Ann Pharmacother. 2013;47:993-998.
- Enke O, New HA, New CH, et al. Anticonvulsants in the treatment of low back pain and lumbar radicular pain: a systematic review and meta-analysis. CMAJ. 2018;190:E786-E93.
- Atkinson JH, Slater MA, Capparelli EV, et al. A randomized controlled trial of gabapentin for chronic low back pain with and without a radiating component. Pain. 2016;157:1499-1507.
- Markman JD, Dworkin RH. Ion channel targets and treatment efficacy in neuropathic pain. J Pain. 2006;7:S38-47.
- Kremer M, Salvat E, Muller A, Yalcin I, Barrot M. Antidepressants and gabapentinoids in neuropathic pain: Mechanistic insights. Neuroscience. 2016;338:183-206.
- Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction. 2016;111:1160-1174.
- Friedman BW, Irizarry E, Solorzano C, et al. Diazepam Is No Better Than Placebo When Added to Naproxen for Acute Low Back Pain. Ann Emerg Med. 2017;70:169-76 e1.
- Fenton MC, Keyes KM, Martins SS, Hasin DS. The role of a prescription in anxiety medication use, abuse, and dependence. Am J Psychiatry. 2010;167:1247-1253.
- Stewart SA. The effects of benzodiazepines on cognition. J Clin Psychiatry. 2005;66 Suppl 2:9-13.
- Leppert W, Malec-Milewska M, Zajaczkowska R, Wordliczek J. Transdermal and Topical Drug Administration in the Treatment of Pain. Molecules. 2018;23.
- McCarberg B, D’Arcy Y. Options in topical therapies in the management of patients with acute pain. Postgrad Med. 2013;125:19-24.
- American College of Rheumatology Ad Hoc Group on Use of S, Nonselective Nonsteroidal Antiinflammatory D. Recommendations for use of selective and nonselective nonsteroidal anti-inflammatory drugs: an American College of Rheumatology white paper. Arthritis Rheum. 2008;59:1058-1073.
- Derry S, Wiffen PJ, Kalso EA, et al. Topical analgesics for acute and chronic pain in adults - an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2017;5:CD008609.
- Massey T, Derry S, Moore RA, McQuay HJ. Topical NSAIDs for acute pain in adults. Cochrane Database Syst Rev. 2010:CD007402.
- Serinken M, Eken C, Tunay K, Golcuk Y. Ketoprofen gel improves low back pain in addition to IV dexketoprofen: a randomized placebo-controlled trial. Am J Emerg Med. 2016;34:1458-1461.
- Wong CS, Wong SH. A new look at trigger point injections. Anesthesiol Res Pract. 2012;2012:492452.
- Kocak AO, Ahiskalioglu A, Sengun E, Gur STA, Akbas I. Comparison of intravenous NSAIDs and trigger point injection for low back pain in ED: A prospective randomized study. Am J Emerg Med. 2019;37(10):1927-1931.
- Hong CZ. Lidocaine injection versus dry needling to myofascial trigger point. The importance of the local twitch response. Am J Phys Med Rehabil. 1994;73:256-263.
- Cleary-Guida MB, Okvat HA, Oz MC, Ting W. A regional survey of health insurance coverage for complementary and alternative medicine: current status and future ramifications. J Altern Complement Med. 2001;7:269-273.
- Gatchel RJ, McGeary DD, McGeary CA, Lippe B. Interdisciplinary chronic pain management: past, present, and future. Am Psychol. 2014;69:119-130.





