Ch. 11 - Vaso-Occlusive Pain
David H. Cisewski, MD, MS | Icahn School of Medicine at Mount Sinai
Jeffrey Glassberg, MD, MA, FACEP | Icahn School of Medicine at Mount Sinai
Sickle cell disease (SCD) is the most common genetic disease in the United States, estimated to affect over 100,000 individuals.1 Of the multitude of potential comorbidities associated with SCD, pain specific to a vaso-occlusive event, vaso-occlusive pain (VOP) (also referred to as a vaso-occlusive crisis or a sickle cell crisis) is the most common presenting complaint in the emergency department.2
Since the first description of the "sickled cell" by Dr. James Herrick in 1910, continued research has advanced our understanding of the pathophysiology of this debilitating disease.3 Yet despite the advances in our knowledge, preventive therapies reduce but do not eliminate vaso-occlusive pain episodes. Today, 109 years after SCD was discovered, there are no optimal therapeutic agents for treating acute SCD pain, and management of these painful episodes remains supportive (note - several preventative agents are being evaluated at the time of this chapter being written). Amid a nationwide opioid epidemic, many health care providers find it challenging to manage acute vaso-occlusive pain among opioid-tolerant sickle cell patients effectively. The purpose of this chapter is to provide evidence-based recommendations on how to safely and effectively manage vaso-occlusive pain presentations in the emergency setting.
PRESENTATION OF VASO-OCCLUSIVE PAIN
Vaso-occlusive pain (VOP) is described as a constellation of muscle, bone, and joint pain that tends to occur most prominently along the marrow-containing bony areas (lower back and legs) as well as the shoulders, upper back, sternum, clavicle, and upper chest.4 The pain is most commonly in multiple locations and consistent with prior crises. The majority of SCD patients experience pain on most days, consistent with the definition of chronic pain. Treatment of chronic pain is not discussed in this chapter. A VOP crisis is defined as the development of acute corporeal pain without a clear secondary cause (eg, trauma, infection). Though an underlying precipitant is often unknown, patients may describe known triggers such as dehydration, stress, sudden exposure to environment temperature extremes or rainfall, infection, physical exertion (sports), and binge alcohol drinking. Vital signs are an inherently unreliable predictor of overall pain and should not be used to assess the degree of severity of a VOP presentation.5,6
VASO-OCCLUSIVE PAIN DIFFERENTIAL DIAGNOSIS
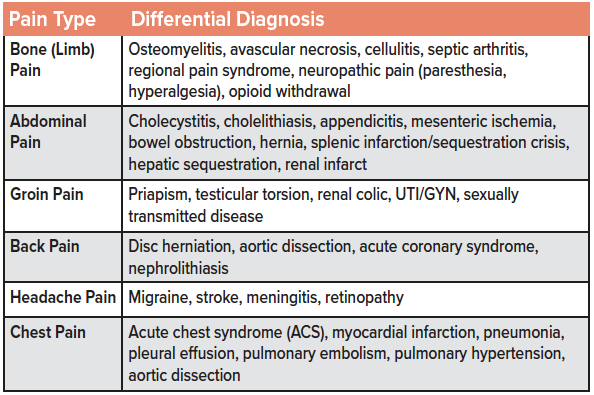
Despite VOP being the leading cause of SCD presentations to the emergency setting, SCD is a diagnosis of exclusion.2,7 It is essential to avoid anchoring on the diagnosis of VOP and to consider non-VOP diagnoses and other life-threatening pathology associated with SCD that may be masked by overwhelming VOP (a listing of items to consider in the differential diagnosis of an SCD patient presenting with concern for VOP is included in the table). Patients with SCD become attuned to their disease state and can often distinguish SCD pain from other pathologies. Discuss the current VOP presentation with the patient and compare the duration, location, and severity of the current episode to previous VOP episodes. Assess for changes in analgesic consumption or medication changes since the last VOP. If a careful history and physical exam does not suggest an acute illness or other non-VOP pathology, laboratory testing and imaging may be safely deferred.7,8
TREATMENT OF VASO-OCCLUSIVE PAIN
The successful management of VOP focuses on 5 key tenets:9
- Early recognition of VOP
- Reduced time to the first-dose analgesic
- Improved care beyond first-dose analgesic regimen
- Maximized patient safety in the ED setting
- Minimized negative provider attitudes toward SCD patients
Aggressive pain management has been shown to prevent the sequelae of unrelieved pain, decrease the duration of pain, decrease hospital admission rate, and reduce the length of stay in the ED.10,11 However, SCD patients have been shown to experience up to 50% longer wait times than the general population for comparable chief complaints.12 While significant efforts are being made to limit opioid utilization in the emergency setting, doing so among the SCD patient population will inevitably lead to inadequate pain control and unnecessary patient suffering. For SCD patients with opioid misuse disorders concerns (sometimes referred to under the non-scientific term: drug-seeking behavior), the decision to restrict opioid use requires a careful and comprehensive review of the patient's record. It is best handled in the outpatient clinical setting. Due to low/moderate levels of baseline chronic pain, recurrent attacks, and frequent ED visits, each vaso-occlusive pain presentation represents a unique, individualized pain experience requiring variable analgesic regimens. This the challenge to emergency providers of how to safely and effectively manage the patient’s pain.
The disease duration, crisis frequency, prior analgesic response, and outpatient management should be considered when initiating a VOP analgesic regimen. Over one-third of SCD patients take long-acting opioids, and just under one-half use short-acting opioids, suggesting a higher prevalence of opioid tolerance.13 Knowing the quantity and frequency of the home analgesic regimen may assist in preventing early undertreatment. Many SCD patients have an individualized VOP regimen created by the patient’s hematologist or pain management specialist, which can be used to address the pain presentation effectively. However, in the absence of such a regimen, a stepwise algorithm including oral, intranasal, nebulized, subcutaneous, and intravenous analgesics may be used to address the pain effectively. Figure 1 represents a novel approach to vaso-occlusive pain management in the emergency setting using a combination of both VOP-specific pharmacologic options as well as analgesic options and routes of administration that have shown efficacy in other pain processes but still require further study for VOP pain. Note: A review of specific institutional guidelines should be completed before implementing this approach or any other off-label analgesic regimen for VOP.
The optimal timing for an analgesic regimen should be within 1 hour of ED arrival or 30 minutes from triage. Flagging SCD patients in triage may accomplish early recognition of VOP for a brief emergency provider evaluation. Once the diagnoses of VOP has been established, efforts should be made toward providing prompt, aggressive analgesic management of the painful crises.7-9,14 Consider a multimodal analgesic approach to VOP that involves several complementary pharmacologic agents targeting different analgesic pathways. This concept of "balanced analgesia" may be used to treat pain while reducing the overall side effect profile of the individual analgesic regimen.15 Early, regular reassessments and analgesic titration until the pain has been optimized are essential to break the pain cycle.
Route of analgesic administration is an important consideration in VOP management. Intranasal (IN), nebulized (NEB), or oral (PO) analgesics may be initiated by skilled nursing staff in triage to reduce mean time to the first-dose analgesia.16 Once triaged to the emergency acute setting, intravenous (IV), intranasal (IN), and subcutaneous (SC) routes of analgesic medications are preferred for rapid pain relief, with nebulized (NEB) formulations as an additional option.8 Oral administration is slow and significantly hindered by first-pass metabolism.17 Intramuscular injections result in variable tissue absorption, pain on injection, and a small but clinically significant risk of tissue necrosis and myofibrosis with repeated injections and should be avoided when possible.18 Although not specific to SCD patients, research has demonstrated that the subcutaneous utilization of opioids is associated with reduced overall opioid exposure with a reduction in pain relief among hospitalized patients suggesting an effective bridge therapy between the emergency department and inpatient admission.19 Provided there are sufficient institutional safety protocols to ensure proper use, patient-controlled analgesia (PCA) with either morphine, fentanyl or hydromorphone may offer a viable alternative to intermittent opioid administration among VOP patient requiring repeated analgesic rounds. For institutions without PCA capability, ensure frequent patient pain reassessments are completed every 30-60 minutes until the pain is controlled. Note that the use of a continuous opioid infusion via PCA in conjunction with on-demand dosing or scheduled intermittent dosing is "per provider discretion," as there is no direct evidence to support the superiority of either intervention.
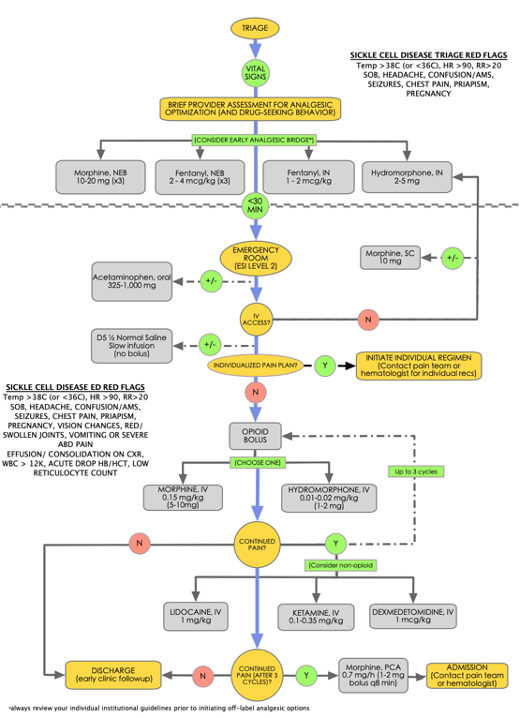
An optimal approach to vaso-occlusive pain focuses on early evaluation and identification, expedited time to first-dose, and rapid reassessments with analgesic re-dosing using a multimodal analgesic regimen as needed to manage pain effectively.
ANALGESIC OPTIONS FOR VASO-OCCLUSIVE PAIN
OPIOID ANALGESIA
Classically, opioids (morphine, hydromorphone, and fentanyl) have all been accepted as the first-line treatment agents for patients suffering from moderate to severe VOP.7
Morphine is considered the gold standard for moderate to severe pain presentations in the emergency setting.20 Consider 0.15 mg/kg IV as a starting dose among opioid-tolerant SCD patients. Despite the general utilization of 0.1 mg/kg IV morphine as standard dosing for moderate to severe pain,21 up to two-thirds of patients of all pain presentations have been shown to have insufficient pain reduction at this dose.22,23 Among opioid-tolerant SCD patients, an even higher percentage may experience subtherapeutic pain relief. Additionally, an anemia-induced increased cardiac output, increased hepatic and renal blood flow, and renal micro-infarctions (causing an increase in glomerular filtration) results in up to a ten-fold increased morphine clearance in SCD patients, further exacerbating an under-dosed morphine regimen.24 Nebulized morphine (10–20 mg) may also be considered a bridge therapy that may be initiated in triage and may have similar efficacy compared to IV morphine.25
Nebulized morphine may effectively reduce pain among SCD patients.26 Furthermore, SC morphine (10 mg) may be considered when IV access remains a hindrance to analgesic relief.21 Morphine PCA pumps among VOP patients have been shown to reduce opioid consumption and overall side effects,28 shorten the ED length of stay,29, and decrease opioid bolus frequency with no observed difference in pain reduction30 when compared to infusions or intermittent injections. Among patients showing improvement with an anticipated discharge disposition, consider a transition from IV to equianalgesic doses (0.3 mg/kg) of oral morphine, which has been shown to decrease the length of ED stay and reduce hospital admissions.31 Note that active morphine metabolites – normally excreted by the kidneys - accumulate in patients with renal function impairment/ renal insufficiency resulting in significant neurotoxicity and respiratory depression.32 In SCD, serum creatinine levels can be adjusted to identify patients with possible undiagnosed acute or chronic renal disease. For patients with HbSS, Sbeta thal zero, a creatinine above 0.8 mg/dL should be considered abnormal. For patients with HbSC or HbSbetha thal plus, a creatinine above 1.0 should be considered abnormal. As patients with SCD are susceptible to acute and chronic kidney injury,33 morphine should be used cautiously or at significant dose reductions in SCD patients with renal disease, on dialysis, or with unknown kidney function.32,34,35
Hydromorphone (HM) is a high-potency opioid, approximately seven-times more potent than morphine.36,37 A dose of 0.01-0.02 mg/kg IV HM (approximately 1–2 mg) may be used for acute VOP presentations. The utilization of a “1+1” dosing protocol - 1 mg IV HM bolus given at presentation followed by a repeat 1 mg IV HM bolus at 15 minutes as needed - has demonstrated a clinically significant pain reduction and limited adverse effects in non-elderly patients presenting with moderate to severe acute pain in the emergency setting.38 While noting that multiple rounds of HM may be needed to break the VOP pain cycle, the safety of the “1+1” protocol is preferred with re-dosing every 15 minutes as needed until the pain is controlled in order to avoid respiratory depression or hemodynamic instability.7 Similar to morphine, the active metabolites of HM are renally excreted and patients with renal impairment may experience neuroexcitatory myoclonus and seizure activity) and respiratory depression.39-41 HM should also be used cautiously or at significant dose reductions in SCD patients with renal disease, on dialysis, or with unknown kidney function.
Research has shown no difference between opioid-related analgesic efficacy or side effects between morphine and hydromorphone at equianalgesic dosing,42 though hydromorphone is associated with increased sedation and euphoria.43 If concern for opioid abuse among patients with VOP, consider reserving HM for patients with morphine-resistant pain or when side effects from large doses of opioids become intolerable.
Fentanyl is an opioid with approximately 100-fold potency compared to morphine allowing for quick onset and short duration bridge to further analgesic treatment.44 A bolus dose of 0.5 mcg/kg IV fentanyl (average 25–50 mcg) may be administered every 10-15 minutes as needed for severe pain presentations in the emergency setting.21 Administration of 2–4 mcg/kg nebulized fentanyl has been shown to effectively reduce time to the first-dose analgesia and may be used up to three times prior to obtaining IV access.45 The utilization of IN fentanyl (INF) has also been shown to increase the rate of discharges when used among the pediatric population.16 Though research is limited to the pediatric population, INF may offer a safe alternative when IV access cannot be obtained46,47, and an improved door-to-analgesia timing is desired.48 As opposed to morphine and hydromorphone, which undergo hepatic metabolism to active metabolites that accumulate in patients with decreased renal function (see above),32,34 fentanyl is metabolized to inactive metabolites, which may offer a safe opioid alternative in SCD patients with decreased renal function.32 However, due to the short analgesic duration of fentanyl, the choice of fentanyl should be limited to scenarios where morphine and hydromorphone are contraindicated, failed to produce acceptable pain relief, or unavailable.
NON-OPIOID ANALGESIA
Ketamine in subdissociative doses (SDK) offers analgesic effects while preserving respiratory and cardiopulmonary stability in the emergency setting.49,50 A sub-anesthetic dose of IV ketamine (0.1–0.35 mg/kg ideal body weight) may be used as a non-opioid alternative for VOP. A number of small case reports and case series have shown IV ketamine is successful in reducing the total opioid consumption and successfully treating refractory VOP pain.51-54 IN ketamine (1 mg/kg) has also been considered an effective delivery alternative based on previous research among the pediatric population46 and is currently being investigated as an adjunct for SCD-related pain.55 Nebulized ketamine (0.5–5 mcg/kg) may also be considered a delivery modality in patients in which IV access cannot be obtained, although data is limited to non-VOP associated pain presentations.56 Ketamine may produce a feeling of unreality, sedation, dizziness, nausea, and nystagmus.50 These side effects can be reduced by administering ketamine as a slow infusion (versus IV push) over 15 minutes.57 When resources are available, consider starting a continuous ketamine infusion in an effort to limit overall opioid use and expedite analgesic relief.
Dexmedetomidine (DXMT) has expanded beyond its traditional role as a sedative in intensive care settings, and cautious optimism surrounds its potential use as an analgesic adjunct in the emergency setting.58 DXMT can be delivered IV or IN with recommended dosing of 0.5–1.0 μg/kg IV (1 to 2 μg/kg IN). 58 DXMT may be beneficial among VOP patients who have developed opioid-induced hyperalgesia or who continue to have pain refractory to an opioid monotherapy regimen as a result of long-term opioid use. 59 DXMT has demonstrated efficacy in severe VOP refractory to ketamine and opioids when used as an adjunct analgesic.60 A small observational analysis showed an initial bolus of 1.0 mcg/kg IV DXMT followed by a 0.5 mcg/kg/hr IV DXMT infusion resulted in successful VOP reduction without hemodynamic compromise among SCD patients admitted for refractory VOP.61 Although promising, further research is needed to confirm the safety and efficacy of DXMT among VOP patients in the emergency setting.
IV lidocaine is an analgesic shown to be efficacious in the treatment of chronic regional pain syndrome,62 post-herpetic neuralgia, and post-spinal cord injury radiculopathy.63 An IV lidocaine bolus (75 mg if < 50 kg, 100 mg if 50-100 kg, and 150 mg if >100 kg) over ten minutes, followed by an infusion of the same amount over 50 minutes, has been shown to result in similar pain relief and reduced side effects compared to morphine for undifferentiated severe acute pain.64 A retrospective study assessing IV lidocaine (1.0–1.3 mg/kg/h) as an analgesic adjunct found a one-third reduction in morphine dosing equivalents (MDEs) with a limited side effect profile among SCD patients.65 Similar to ketamine, further research is needed to confirm the safety and efficacy of lidocaine among VOP patients in the emergency setting.
Acetaminophen (APAP, paracetamol) is often used as a first-line agent for acute pain presentations of mild to moderate intensity in the ED-setting.66,67 Although the VOP presentation is typically moderate to severe in pain intensity, acetaminophen may be considered an adjunct in a multimodal analgesic approach to VOP. Administration of 325 to 1,000 mg PO acetaminophen every 4–6 hours (maximum 4 g/day) is a recommended regimen for acute pain presentation. 21
NON-STEROIDAL ANTI-INFLAMMATORY DRUGS
While not absolutely contraindicated, NSAID use should be limited in the treatment of VOP. Patients with SCD are susceptible to acute and chronic kidney injury33, and creatinine levels may not accurately reflect glomerular filtration rate in SCD.69 Low-level renal microinfarcts seen in SCD lead to hyposthenuria and supra-normal proximal tubular function, which may result in a normal creatinine level giving the false reassurance of normal renal function.7 It is safe to assume that all patients with SCD have at least a mild degree of renal dysfunction, and as such, the use of nephrotoxic analgesics should be minimized. And if NSAIDs are used, they should be given at the analgesic ceiling doses and to be prescribed for the shortest course.
NITROUS OXIDE (N2O) is an odorless gas with a fast onset and a short half-life that has typically been used for procedural anesthesia and analgesia.70,71 Analgesic N2O administered as a combined of 50% oxygen – 50% nitrous oxide72 offers analgesia with an average onset of 3-5 minutes (recovery occurring within approximately five minutes of discontinuation).71 Although data specific to SCD patients is limited, N2O research in the pediatric population has demonstrated efficacy for bone and joint procedures,73 laceration repairs, abscess drainage, foreign body removal, and urine catheterization placement,70,74 suggesting efficacy in pain reduction. Further, a small case report study has suggested N2O may be effective in resolving priapism associated with VOP.75 N2O is particularly advantageous among SCD patients where IV access is difficult to obtain. Further data is needed to understand the safety and efficacy of N2O among VOP patients in the emergency setting.
SPECIAL TREATMENT CONSIDERATIONS
ANTIHISTAMINES
Pruritis is a common dose-dependent side effect of morphine and HM use.37 This pruritis is mu-receptor-mediated in 90% of cases.76 If pruritus becomes intolerable, administer 25 to 50 mg PO diphenhydramine rather than intravenous as the latter potentiate sedation and euphoria while offering limited additional pruritic relief.77
SUPPLEMENTAL OXYGEN
There are no specific guidelines nor evidence that the administration of supplemental oxygen reduces VOP recovery time or admission rate. There is a theoretical concern that the administration of supplemental oxygen may further inhibit the release of hypoxia-induced transcription factors (HIF) necessary for erythropoietin production, further delaying essential hematopoiesis and delaying recovery rate.78 At our center, patients with VOP are given supplemental oxygen to keep saturation above 95% with down-titration among patients who achieve consistent saturation of 100%. An incentive spirometer should be utilized for all patients requiring admission as a simple, low-cost intervention to prevent pulmonary infiltrates, atelectasis, and acute chest syndrome development.7,79
NITRIC OXIDE (NO)
NO functions as both a vasodilator and an inhibitor of platelet aggregation and cell-adhesion that attribute to the pain of a VOP.80 Despite the potential for analgesic relief secondary to NO-induced vasodilation, research has demonstrated no difference in pain reduction, crises duration, or opioid consumption in nitric oxide versus placebo among VOP patients. 80 Further data is needed to understand the safety and efficacy of NO among subsets of VOP patients in the emergency setting.
FLUIDS
Although dehydration represents a common precipitant of VOP, overhydration with isotonic fluid boluses may increase the likelihood of further sequelae such as atelectasis, pulmonary infiltrates, and acute chest syndrome.7,81,82 Normal saline may also promote hyperchloremic metabolic acidosis.7 worsen pain control, and increase rates of admission among VOP patients.81,82 Nevertheless, lowering the serum osmolality may prevent further erythrocyte sickling and offset dehydration and may be used cautiously among volume-depleted patients.83 Recommendations are to initiate a slow D5 ½ normal saline infusion at a maintenance rate in patients who appear to have dehydration-induced VOP.7
DESMOPRESSIN (DDAVP)
DDAVP has been speculated to improve VOP recovery by increasing fluid retention and increasing vascular fluid load in patients with concern for dehydration. However, this suggestion is anecdotal, and no evidence of decreased hospital stay nor additional analgesic benefit has been shown. Given the key role that platelets play in initiating vaso-occlusion, we do not recommend DDAVP at this time.
MAGNESIUM
Magnesium has been suggested to improve patient outcome in SCD VOP through its vasodilatory and anti-inflammatory properties.84 However, research has shown that the addition of IV magnesium to a treatment regimen does not shorten the length of stay, reduce opioid use, reduce pain, nor improve quality of life among patients with VOP.85,86
EXCHANGE OR SIMPLE TRANSFUSION
Exchange/simple transfusion is not indicated for the acute treatment of uncomplicated VOP. Any patient deteriorating despite aggressive VOP management may be considered for exchange transfusion following a hematology consult.7
DISPOSITION OF VASO-OCCLUSIVE PAIN PRESENTATIONS
During serial reassessments, providers are encouraged to talk with the patient and make a shared decision based on the goals of care and the desired outcome. The primary goal of emergency pain management is not zero pain, but a reduction in pain to an acceptable level that will allow for a safe discharge with a return to the patients’ daily activities.87
Patients who demonstrate sufficient pain reduction and no evidence of further SCD sequelae may be discharged home with close outpatient follow up and return precautions. The emergency department is a time and resource-constrained setting, which may limit a full assessment of the patient’s analgesic needs. A comprehensive pain assessment is best performed in an outpatient clinic setting.9 Patients who do not have established outpatient care should be set up with a hematologist (or non-hematology sickle cell specialist) appointment for further symptom management. For patients requiring an outpatient analgesic regimen, emergency providers should limit prescribing opioids beyond a short, 3-day course and should instead defer the management to outpatient clinics for close follow up. Optimally a single provider who knows and understands the patient’s pain profile should be used to tailor an individualized pain management regimen. Additionally, non-pharmacologic pain management strategies such as transcutaneous electric nerve stimulation (TENS), relaxation techniques, massage/physical therapy, and distraction techniques such as virtual reality may be effective analgesic adjuncts to be used in the outpatient setting highlighting the importance of outpatient follow-up for customized pain management.88-90
Patients who continue to have pain refractory to intravenous hydration and aggressive pain control may ultimately require hospital admission. Though no standard protocol or guidelines provide a specific determinant for admission, many providers will admit VOP patients after three doses of IV opioids with insufficient pain relief.
ADDITIONAL VASO-OCCLUSIVE MANAGEMENT CONSIDERATIONS
Identification of high-risk patients more likely to return is key to preventing frequent utilization of the emergency department setting for their pain crises. Patients aged 18-30 years old have the highest rate of ED utilization for VOP and mortality rate from SCD-related comorbidities.91 A significant correlation has also been shown between SCD-patient opioid use and symptom burden, disease-associated stress, negative coping habit, and quality of life (both physical and mental).13 Providing additional resources and social support for these individuals – particularly those averaging more than one visit per month - is essential to prevent high-utilization of emergency services and hospital readmission.92 Frequent utilizers of emergency department services should be offered a further discussion with the hospital social work staff who may be able to identify underlying barriers and social challenges to outpatient SCD management.
DEEP DIVE: IMPLEMENTING AN ED-BASED VASO-OCCLUSIVE PAIN MANAGEMENT PROGRAM
VOP treatment is hindered by delayed triage recognition, extended ED wait times, and negative attitudes by health care staff who may see these VOP presenters as drug-seeking opioid addicts, manipulative, and uncooperative with health care staff.93 Research has shown that over half of ED physicians have a perceived belief that at least 20% of SCD patients are addicted to opioids.94 Similar beliefs account for the reluctance to deliver repeat analgesic doses to SCD patients experiencing a painful crisis.95 In reality, only a small subset of SCD patients compromise a large percentage of the overall ED visits, creating negative perception of VOP presenters.96
The successful management and disposition of VOP patients is dependent on health care professional (HCP) attitudes toward this specific patient population. Use of the term "sickler" has been associated with negative attitudes towards patients with SCD and should also be avoided.97 Consider terms such as "vaso-occlusive patients" or "vaso-occlusive events" as an alternative way to refer to these patients and their ED presentation. Reducing negative attitudes toward patients through educational initiatives on disease pathophysiology and providing evidence that counters biases and stereotypes are essential to optimizing VOP patient care. Studies have demonstrated that a short video-based education tutorial may result in a significant improvement in HCP attitudes toward patients in pain.98,99 Example training videos can be found on the Duke Emergency Department Sickle Disease program.
Initiatives focused on reducing time to first dose of analgesic medication are essential to optimizing VOP management. Develop a protocol for triage management (similar to a stroke code) that will limit the duration in which these patients will be without analgesia. Consider incorporating alternatives to intravenous use for triage analgesic interventions such as nebulized, and intranasal analgesics. Open the discussion to the hospital pharmacy staff and discuss the possibilities of initiating intranasal or nebulized analgesic intervention in the triage area. Along with pharmacy support, a successful plan should involve HCP’s across the entire spectrum, including triage and emergency nurses, residents, physicians, nurse practitioners, and physician assistants. Educational resources and training modules for all levels of care can be utilized through the Duke Sickle Cell Emergency program.
If available at your institution, emergency physicians can be given contact information for the care management team that handles individuals when ultra-utilization or drug-seeking behavior becomes a concern.9 Hospitals with a dedicated acute care unit and specialized knowledge of VOP patients are more aggressive with pain treatment and increased first time dosing, which results in increased pain reduction and adecreased hospital admission rate compared to regular ED care.100 Additionally, patients treated on a patient-specific opioid regimen received greater pain relief, had less nausea and vomiting, and an over 20% reduction in admission rate.11 When available, consult hematology or pain management services to optimize treatment among more challenging patients.
Finally, efforts should be made to improve ED patient safety for SCD patients. SCD pain is a diagnosis of exclusion, and it is important to avoid cognitive biases and early anchoring by ensuring that incoming trainees are aware of the differential diagnoses that must be ruled out during the initial SCD evaluation as well as providing safer analgesic practices. Always ensure pulse oximetry and cardiac monitoring are available for patient safety during escalating analgesic management phase, particularly when high-dose opioids are being utilized.
SUMMARY
VOP is the most common chief complaint of SCD patients presenting to the emergency department. A focus on early recognition and analgesic initiation, along with a comprehensive review of the VOP differential diagnoses is essential to effectively managing the pain and limiting further disease sequelae. Typical analgesic regimens may be ineffective among opioid-tolerant SCD patients who typically require higher starting doses for these acute pain presentations. By utilizing a multimodal analgesic approach that begins in triage and involves early, serial reassessments and re-administration, emergency departments can limit admission rates and ED length of stay. Efforts made to educate the entire spectrum of emergency health care professionals to dispel myths and misconceptions that hinder patient care is essential to improving patient-provider communication and analgesic management.
References
- CDC. Data & Statistics on Sickle Cell Disease. 2017 August 9, 2017; Available from: https://www.cdc.gov/NCBDDD/sicklecell/data.html.
- Smith, W.R., et al., Daily assessment of pain in adults with sickle cell disease. Ann Intern Med, 2008. 148(2): p. 94-101.
- Herrick, J.B., Peculiar elongated and sickle-shaped red blood corpuscles in a case of severe anemia. JAMA, 2014. 312(10): p. 1063.
- McClish, D.K., et al., Pain site frequency and location in sickle cell disease: the PiSCES project. Pain, 2009. 145(1-2): p. 246-51.
- Dayoub, E.J. and A.B. Jena, Does Pain Lead to Tachycardia? Revisiting the Association Between Self-reported Pain and Heart Rate in a National Sample of Urgent Emergency Department Visits. Mayo Clin Proc, 2015. 90(8): p. 1165-6.
- Ernst, A.A., et al., Blood pressure in acute vaso-occlusive crises of sickle cell disease. South Med J, 2000. 93(6): p. 590-2.
- Glassberg, J., Evidence-based management of sickle cell disease in the emergency department. Emerg Med Pract, 2011. 13(8): p. 1-20; quiz 20.
- Lovett, P.B., H.P. Sule, and B.L. Lopez, Sickle Cell Disease in the Emergency Department. Hematol Oncol Clin North Am, 2017. 31(6): p. 1061-1079.
- Glassberg, J.A., Improving Emergency Department-Based Care of Sickle Cell Pain. Hematology Am Soc Hematol Educ Program, 2017. 2017(1): p. 412-417.
- Benjamin, L.J., G.I. Swinson, and R.L. Nagel, Sickle cell anemia day hospital: an approach for the management of uncomplicated painful crises. Blood, 2000. 95(4): p. 1130-6.
- Tanabe, P., et al., A randomized controlled trial comparing two vaso-occlusive episode (VOE) protocols in sickle cell disease (SCD). Am J Hematol, 2018. 93(2): p. 159-168.
- Haywood, C., Jr., et al., The impact of race and disease on sickle cell patient wait times in the emergency department. Am J Emerg Med, 2013. 31(4): p. 651-6.
- Smith, W.R., et al., Daily home opioid use in adults with sickle cell disease: The PiSCES project. J Opioid Manag, 2015. 11(3): p. 243-53.
- Solomon, L.R., Treatment and prevention of pain due to vaso-occlusive crises in adults with sickle cell disease: an educational void. Blood, 2008. 111(3): p. 997-1003.
- Hosseininejad, S.M., et al., Efficacy and Safety of Combination Therapy with Ketorolac and Morphine in Patient with Acute Renal Colic; A Triple-Blind Randomized Controlled Clinical Trial. Bull Emerg Trauma, 2017. 5(3): p. 165-170.
- Kavanagh, P.L., et al., Improving the Management of Vaso-Occlusive Episodes in the Pediatric Emergency Department. Pediatrics, 2015. 136(4): p. e1016-25.
- Glare, P.A. and T.D. Walsh, Clinical pharmacokinetics of morphine. Ther Drug Monit, 1991. 13(1): p. 1-23.
- Greenblatt, D.J. and M.D. Allen, Intramuscular injection-site complications. JAMA, 1978. 240(6): p. 542-4.
- Ackerman, A.L., et al., Association of an Opioid Standard of Practice Intervention With Intravenous Opioid Exposure in Hospitalized Patients. JAMA Intern Med, 2018. 178(6): p. 759-763.
- Ruiz-Garcia, V. and E. Lopez-Briz, Morphine remains gold standard in breakthrough cancer pain. BMJ, 2008. 337: p. a3104.
- Motov, S. and R. Hossain, Tarascon Pain Pocketbook. Vol. 1. 2018, Burlington, MA: Jones and Bartlett Learning LLC.
- Bijur, P.E., M.K. Kenny, and E.J. Gallagher, Intravenous morphine at 0.1 mg/kg is not effective for controlling severe acute pain in the majority of patients. Ann Emerg Med, 2005. 46(4): p. 362-7.
- Birnbaum, A., et al., Randomized double-blind placebo-controlled trial of two intravenous morphine dosages (0.10 mg/kg and 0.15 mg/kg) in emergency department patients with moderate to severe acute pain. Ann Emerg Med, 2007. 49(4): p. 445-53, 453 e1-2.
- Darbari, D.S., et al., Increased clearance of morphine in sickle cell disease: implications for pain management. J Pain, 2011. 12(5): p. 531-8.
- Grissa, M.H., et al., Efficacy and safety of nebulized morphine given at 2 different doses compared to IV titrated morphine in trauma pain. Am J Emerg Med, 2015. 33(11): p. 1557-61.
- Ballas, S.K., E.R. Viscusi, and K.R. Epstein, Management of acute chest wall sickle cell pain with nebulized morphine. Am J Hematol, 2004. 76(2): p. 190-1.
- Telfer, P., et al., Intranasal diamorphine for acute sickle cell pain. Arch Dis Child, 2009. 94(12): p. 979-80.
- van Beers, E.J., et al., Patient-controlled analgesia versus continuous infusion of morphine during vaso-occlusive crisis in sickle cell disease, a randomized controlled trial. Am J Hematol, 2007. 82(11): p. 955-60.
- Gonzalez, E.R., et al., Intermittent injection vs patient-controlled analgesia for sickle cell crisis pain. Comparison in patients in the emergency department. Arch Intern Med, 1991. 151(7): p. 1373-8.
- Santos, J., et al., Patient Controlled Analgesia for Adults with Sickle Cell Disease Awaiting Admission from the Emergency Department. Pain Res Manag, 2016. 2016: p. 3218186.
- Campos, J., et al., Treatment of the acute sickle cell vaso-occlusive crisis in the Emergency Department: a Brazilian method of switching from intravenous to oral morphine. Eur J Haematol, 2014. 93(1): p. 34-40.
- Dean, M., Opioids in renal failure and dialysis patients. J Pain Symptom Manage, 2004. 28(5): p. 497-504.
- Nath, K.A. and R.P. Hebbel, Sickle cell disease: renal manifestations and mechanisms. Nat Rev Nephrol, 2015. 11(3): p. 161-71.
- Pham, P.C., et al., 2017 update on pain management in patients with chronic kidney disease. Clin Kidney J, 2017. 10(5): p. 688-697.
- Ishida, J.H., et al., Opioid Analgesics and Adverse Outcomes among Hemodialysis Patients. Clin J Am Soc Nephrol, 2018. 13(5): p. 746-753.
- Sarhill, N., D. Walsh, and K.A. Nelson, Hydromorphone: pharmacology and clinical applications in cancer patients. Support Care Cancer, 2001. 9(2): p. 84-96.
- Mazer-Amirshahi, M., S. Motov, and L.S. Nelson, Hydromorphone use for acute pain: Misconceptions, controversies, and risks. J Opioid Manag, 2018. 14(1): p. 61-71.
- Chang, A.K., et al., Safety and efficacy of rapid titration using 1mg doses of intravenous hydromorphone in emergency department patients with acute severe pain: the “1+1” protocol. Ann Emerg Med, 2009. 54(2): p. 221-5.
- Trescot, A.M., et al., Opioid pharmacology. Pain Physician, 2008. 11(2 Suppl): p. S133-53.
- Smith, M.T., Neuroexcitatory effects of morphine and hydromorphone: evidence implicating the 3-glucuronide metabolites. Clin Exp Pharmacol Physiol, 2000. 27(7): p. 524-8.
- Smith, H.S., Opioid metabolism. Mayo Clin Proc, 2009. 84(7): p. 613-24.
- Hong, D., P. Flood, and G. Diaz, The side effects of morphine and hydromorphone patient-controlled analgesia. Anesth Analg, 2008. 107(4): p. 1384-9.
- Bryson, E.O., The anesthetic implications of illicit opioid abuse. Int Anesthesiol Clin, 2011. 49(1): p. 67-78.
- Burns, S.M., C.W. Cunningham, and S.L. Mercer, DARK Classics in Chemical Neuroscience: Fentanyl. ACS Chem Neurosci, 2018.
- Thompson, J.P. and D.F. Thompson, Nebulized Fentanyl in Acute Pain: A Systematic Review. Ann Pharmacother, 2016. 50(10): p. 882-91.
- Reynolds, S.L., et al., Randomized Controlled Feasibility Trial of Intranasal Ketamine Compared to Intranasal Fentanyl for Analgesia in Children with Suspected Extremity Fractures. Acad Emerg Med, 2017. 24(12): p. 1430-1440.
- Rech, M.A., et al., When to Pick the Nose: Out-of-Hospital and Emergency Department Intranasal Administration of Medications. Ann Emerg Med, 2017. 70(2): p. 203-211.
- Schaefer, J.A. and T.J. Mlekoday, Time to opioid administration after implementation of an intranasal fentanyl protocol. Am J Emerg Med, 2015. 33(12): p. 1805-7.
- Yeaman, F., et al., Sub-dissociative-dose intranasal ketamine for moderate to severe pain in adult emergency department patients. Emerg Med Australas, 2014. 26(3): p. 237-42.
- Motov, S., et al., Continuous Intravenous Sub-Dissociative Dose Ketamine Infusion for Managing Pain in the Emergency Department. West J Emerg Med, 2018. 19(3): p. 559-566.
- Tawfic, Q.A., A.S. Faris, and R. Kausalya, The role of a low-dose ketamine-midazolam regimen in the management of severe painful crisis in patients with sickle cell disease. J Pain Symptom Manage, 2014. 47(2): p. 334-40.
- Uprety, D., A. Baber, and M. Foy, Ketamine infusion for sickle cell pain crisis refractory to opioids: a case report and review of literature. Ann Hematol, 2014. 93(5): p. 769-71.
- Nobrega, R., et al., Patient characteristics affect the response to ketamine and opioids during the treatment of vaso-occlusive episode-related pain in sickle cell disease. Pediatr Res, 2018. 83(2): p. 445-454.
- Palm, N., et al., Low-Dose Ketamine Infusion for Adjunct Management during Vaso-occlusive Episodes in Adults with Sickle Cell Disease: A Case Series. J Pain Palliat Care Pharmacother, 2018. 32(1): p. 20-26.
- Young, J.R., et al., Subdissociative intranasal ketamine plus standard pain therapy versus standard pain therapy in the treatment of paediatric sickle cell disease vaso-occlusive crises in resource-limited settings: study protocol for a randomised controlled trial. BMJ Open, 2017. 7(7): p. e017190.
- Abdel-Ghaffar, H.S., et al., [Preemptive nebulized ketamine for pain control after tonsillectomy in children: randomized controlled trial]. Rev Bras Anestesiol, 2019. 69(4): p. 350-357.
- Motov, S., et al., A prospective randomized, double-dummy trial comparing IV push low dose ketamine to short infusion of low dose ketamine for treatment of pain in the ED. Am J Emerg Med, 2017. 35(8): p. 1095-1100.
- Mahmoud, M. and K.P. Mason, Dexmedetomidine: review, update, and future considerations of paediatric perioperative and periprocedural applications and limitations. Br J Anaesth, 2015. 115(2): p. 171-82.
- Brush, D.E., Complications of long-term opioid therapy for management of chronic pain: the paradox of opioid-induced hyperalgesia. J Med Toxicol, 2012. 8(4): p. 387-92.
- Sheehy, K.A., et al., Dexmedetomidine as an Adjuvant to Analgesic Strategy During Vaso-Occlusive Episodes in Adolescents with Sickle-Cell Disease. Pain Pract, 2015. 15(8): p. E90-7.
- Phillips, W.J., et al., Dexmedetomidine relieves pain associated with acute sickle cell crisis. J Pain Symptom Manage, 2007. 34(4): p. 346-9.
- Wallace, M.S., et al., Concentration-effect relationship of intravenous lidocaine on the allodynia of complex regional pain syndrome types I and II. Anesthesiology, 2000. 92(1): p. 75-83.
- Tremont-Lukats, I.W., P.R. Hutson, and M.M. Backonja, A randomized, double-masked, placebo-controlled pilot trial of extended IV lidocaine infusion for relief of ongoing neuropathic pain. Clin J Pain, 2006. 22(3): p. 266-71.
- Clattenburg, E.J., et al., Intravenous Lidocaine Provides Similar Analgesia to Intravenous Morphine for Undifferentiated Severe Pain in the Emergency Department: A Pilot, Unblinded Randomized Controlled Trial. Pain Med, 2018.
- Nguyen, N.L., et al., Intravenous Lidocaine as an Adjuvant for Pain Associated with Sickle Cell Disease. J Pain Palliat Care Pharmacother, 2015. 29(4): p. 359-64.
- Chang, A.K., et al., Effect of a Single Dose of Oral Opioid and Nonopioid Analgesics on Acute Extremity Pain in the Emergency Department: A Randomized Clinical Trial. JAMA, 2017. 318(17): p. 1661-1667.
- Optimizing the Treatment of Acute Pain in the Emergency Department. Ann Emerg Med, 2017. 70(3): p. 446-448.
- Barden, J., et al., Single dose oral paracetamol (acetaminophen) for postoperative pain. Cochrane Database Syst Rev, 2004(1): p. CD004602.
- Marouf, R., et al., Comparison of renal function markers in Kuwaiti patients with sickle cell disease. J Clin Pathol, 2006. 59(4): p. 345-51.
- Huang, C. and N. Johnson, Nitrous Oxide, From the Operating Room to the Emergency Department. Curr Emerg Hosp Med Rep, 2016. 4: p. 11-18.
- Pasaron, R., et al., Nitrous oxide procedural sedation in non-fasting pediatric patients undergoing minor surgery: a 12-year experience with 1,058 patients. Pediatr Surg Int, 2015. 31(2): p. 173-80.
- American Society of Anesthesiologists Task Force on, S. and N.-A. Analgesia by, Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology, 2002. 96(4): p. 1004-17.
- Reinoso-Barbero, F., et al., Equimolar nitrous oxide/oxygen versus placebo for procedural pain in children: a randomized trial. Pediatrics, 2011. 127(6): p. e1464-70.
- Tobias, J.D., Applications of nitrous oxide for procedural sedation in the pediatric population. Pediatr Emerg Care, 2013. 29(2): p. 245-65.
- Greenwald, M. and C. Morris, Nitrous Oxide Gas May be a Promising Therapy for Acute Priapism in Patients with Sickle Cell Disease: A Case Report. Blood, 2017. 130: p. 4802.
- Kjellberg, F. and M.R. Tramer, Pharmacological control of opioid-induced pruritus: a quantitative systematic review of randomized trials. Eur J Anaesthesiol, 2001. 18(6): p. 346-57.
- Kumar, K. and S.I. Singh, Neuraxial opioid-induced pruritus: An update. J Anaesthesiol Clin Pharmacol, 2013. 29(3): p. 303-7.
- Frede, S., et al., Oxygen-regulated expression of the erythropoietin gene in the human renal cell line REPC. Blood, 2011. 117(18): p. 4905-14.
- Bellet, P.S., et al., Incentive spirometry to prevent acute pulmonary complications in sickle cell diseases. N Engl J Med, 1995. 333(11): p. 699-703.
- Gladwin, M.T., et al., Nitric oxide for inhalation in the acute treatment of sickle cell pain crisis: a randomized controlled trial. JAMA, 2011. 305(9): p. 893-902.
- Carden, M.A., et al., Variations in pediatric emergency medicine physician practices for intravenous fluid management in children with sickle cell disease and vaso-occlusive pain: A single institution experience. Pediatr Blood Cancer, 2018. 65(1).
- Carden, M.A., et al., Normal Saline Bolus Use in Pediatric Emergency Departments is Associated with Worse Pain Control in Children with Sickle Cell Anemia and Vaso-occlusive Pain. Am J Hematol, 2019.
- Clark, M.R., et al., Influence of red cell water content on the morphology of sickling. Blood, 1980. 55(5): p. 823-30.
- Yang, Z.W., et al., Mg(2+)-induced endothelium-dependent relaxation of blood vessels and blood pressure lowering: role of NO. Am J Physiol Regul Integr Comp Physiol, 2000. 278(3): p. R628-39.
- Brousseau, D.C., et al., A multicenter randomized controlled trial of intravenous magnesium for sickle cell pain crisis in children. Blood, 2015. 126(14): p. 1651-7.
- Goldman, R.D., et al., Intravenous magnesium sulfate for vaso-occlusive episodes in sickle cell disease. Pediatrics, 2013. 132(6): p. e1634-41.
- Lee, T.H., Zero Pain Is Not the Goal. JAMA, 2016. 315(15): p. 1575-7.
- Wang, W.C., S.L. George, and J.A. Wilimas, Transcutaneous electrical nerve stimulation treatment of sickle cell pain crises. Acta Haematol, 1988. 80(2): p. 99-102.
- Majumdar, S., et al., The use and effectiveness of complementary and alternative medicine for pain in sickle cell anemia. Complement Ther Clin Pract, 2013. 19(4): p. 184-7.
- Agrawal, A.K., et al., Virtual reality as complementary pain therapy in hospitalized patients with sickle cell disease. Pediatr Blood Cancer, 2019. 66(2): p. e27525.
- Brousseau, D.C., et al., Acute care utilization and rehospitalizations for sickle cell disease. JAMA, 2010. 303(13): p. 1288-94.
- Koch, K.L., et al., Intensive management of high-utilizing adults with sickle cell disease lowers admissions. Am J Hematol, 2015. 90(3): p. 215-9.
- Jenerette, C.M., et al., Does Attendance at a Sickle Cell Educational Conference Improve Clinician Knowledge and Attitude Toward Patients with Sickle Cell Disease? Pain Manag Nurs, 2016. 17(3): p. 226-34.
- Shapiro, B.S., et al., Sickle cell-related pain: perceptions of medical practitioners. J Pain Symptom Manage, 1997. 14(3): p. 168-74.
- Glassberg, J.A., et al., Emergency provider analgesic practices and attitudes toward patients with sickle cell disease. Ann Emerg Med, 2013. 62(4): p. 293-302 e10.
- Aisiku, I.P., et al., Comparisons of high versus low emergency department utilizers in sickle cell disease. Ann Emerg Med, 2009. 53(5): p. 587-93.
- Glassberg, J., et al., Among emergency physicians, use of the term “Sickler” is associated with negative attitudes toward people with sickle cell disease. Am J Hematol, 2013. 88(6): p. 532-3.
- Puri Singh, A., et al., Improving Emergency Providers’ Attitudes Toward Sickle Cell Patients in Pain. J Pain Symptom Manage, 2016. 51(3): p. 628-32 e3.
- Haywood, C., Jr., et al., A video-intervention to improve clinician attitudes toward patients with sickle cell disease: the results of a randomized experiment. J Gen Intern Med, 2011. 26(5): p. 518-23.
- Molokie, R.E., et al., Opioid doses and acute care utilization outcomes for adults with sickle cell disease: ED versus acute care unit. Am J Emerg Med, 2018. 36(1): p. 88-92.





