Ch. 15 - Wilderness Pain Management
Adam D. Hill, MD | Icahn School of Medicine at Mount Sinai/Elmhurst
Treating pain in wilderness or remote environments where access to advanced care is limited creates a unique situation not often encountered by physicians. The United States National Park Service (USNPS) estimates over 300 million annual visitors to its park system each year and averages ten search and rescue incidents per day,1-3 many of which involve acute injuries. With that in mind, it is important for physicians to have a coherent approach to pain in wilderness (austere) settings.
APPROACH TO PAIN IN THE WILD
Whether traveling alone in the backcountry or serving as the physician for a large expedition, critical factors in managing pain in the outdoor setting are anticipation, preparation, and versatility. As there is no one specific pain presentation in the wild - any and all types of pathology may occur - physicians must anticipate a trend toward specific injuries based on location as well as the activity being undertaken and prepare their medical kit to reflect this. As weight, size, and durability of medications are imperative in the field, using analgesic modalities that can treat multiple painful conditions is ideal. The focus must be given not only to stabilizing and treating injuries but also to optimizing patient status in preparation for a potentially prolonged evacuation.
COMMON PAIN PRESENTATIONS
The majority of painful conditions in the wild will revolve around musculoskeletal, skin, and soft-tissue injuries.4 Sprains, strains, fractures, lacerations, burns, head injuries, and blisters will frequently be encountered. Other organ systems are at risk as well, such as ophthalmic (corneal abrasions, conjunctivitis, snow blindness), otic (otitis media, otitis externa), dental (abscess, fracture, dentalgia), neurologic (headache), integumentary (cellulitis, abscess, dermatitis, in-grown nails, subungual hematoma, paronychia), gastrointestinal, and cardiovascular, to name a few. Recognizing the therapeutic overlap between many of these conditions allows the physician to provide optimal care while being mindful of the inherent size and weight limitations of any wilderness endeavor.
PAIN ASSESSMENT
Scene Safety, Identification, Consent
The first step in caring for a patient in the wild is to ensure the surrounding area is free of hazards to the responding physician, thus preventing the creation of additional patients. Identify oneself as a physician with the ability to aid the patient. For any treatment or management being conducted, ensure verbal consent is obtained prior to providing care. Similar to the emergency department (ED), there is no implied consent in the outdoors save for situations in which the individual is a minor, lacks capacity, or is unresponsive, at which point consent is implied.
PAIN MANAGEMENT
Non-Pharmacologic Analgesic approaches to pain should always be undertaken first. This includes several basic first-aid maneuvers: immobilization, elevation, and the splinting or wrapping of any injured extremity can reduce pain and assist in patient evacuation; addressing a “hot-spot” on a hiker’s foot to prevent blisters may further aid in ambulation efforts; placing a superficial burn under cold running water may provide immediate pain relief and improved healing times.5 The indirect application of snow or ice to burns can be used in cold environments in which hypothermia is not a concern. Reassuring the patient with a calm voice and encouraging deep and slow breathing techniques (DSB) can also aid in pain management.6
Pharmacologic Analgesia
As the types of pain etiologies encountered in the wild can be as varied as the terrain, it is impossible to recommend a universal pharmacologic approach that encompasses all pain presentations. However, there are fundamental ideas that can be applied to most painful encounters in the austere setting. Attention should be paid to identifying and treating any possible underlying causes of pain, such as infection or exposure, in addition to the pain itself.
Nonsteroidal Anti-inflammatory Drugs (NSAIDs)
Ibuprofen 400 mg oral with acetaminophen 1,000 mg oral can produce the analgesic effect of commonly used oral opioids/acetaminophen combinations.7 In the absence of contraindications, this should be the starting point in the treatment of almost every painful condition, with re-dosing every 6 hours as needed. Meloxicam 15 mg oral once every 24 hours is the preferred NSAID for battlefield medicine, but, as it requires a prescription, it may not be available in this setting.8 Additionally, diclofenac 50-75 mg every 12 hours, and naproxen 500 mg every 12 hours may be considered as effective alternatives to ibuprofen.
Aspirin (ASA)
An often-overlooked medication due to its intricate association with cardiovascular emergencies, ASA can be used at doses of 650mg every 4-6 hours for pain control.9 The combination ASA/APAP/Caffeine (AAC) has demonstrated analgesic efficacy for tension-type headaches10 and migraines11 and may be beneficial for other pain presentations though GI tolerance often limits use.12 ASA has the additional advantage of being cardioprotective if the pain is thought to be due to myocardial ischemia or infarct, given at a dose of 325 mg (chewed, non-enteric coated preparation). As ASA is an NSAID, it should not be administered along with other NSAIDs and is contraindicated in trauma patients at risk for bleeding or hemorrhage due to its antiplatelet activity.
Ketamine
Ketamine has increasingly become an alternative therapy for pain control in victims of trauma, including Tactical Combat Casualty Care (TCCC) Guidelines, who released a statement supporting its use.8 Ketamine has the benefit of being administered IV, IO, IM, SQ, inhaled, or IN. In sub-dissociative dosing (SDK), 0.5-1 mg/kg IM, 1 mg/kg IN, or 0.1-0.3 mg/kg IV/IO/SQ as a slow infusion over 15 minutes13 every 20-30 minutes titrated to pain relief or development of severe psychoperceptual side effects may be used to optimize pain relief. The analgesic effect of ketamine may be amplified when given in combination with an opioid.14 At higher doses, 3-4 mg/kg (~300-500 mg) IM or 1-1.5 mg/kg (~100 mg) IV/IO, it provides sedation through its dissociative properties. This increases its versatility in the field, allowing a physician to manage the severely agitated patients, to provide brief sedation for painful procedures, or to create a prolonged state of sedation with repeat dosing to enable evacuation/extrication from dangerous terrain.15 IN dosing at 3-9 mg/kg has been described for effective pediatric sedation.16,17 When dissociative doses are being administered via IV/IO, they should be given over 1-2 minutes to prevent laryngeal spasm. It should be noted that emergence reaction is a common occurrence with dissociation and can be mitigated with the administration of a benzodiazepine, such as midazolam 2 mg or diazepam 2-5 mg IV/IO18,19 or as a slow infusion over 15 minutes if time allows.13
Benzodiazepines
Though benzodiazepines contain no intrinsic analgesic properties, they may work in tandem with analgesics in certain situations. Diazepam 2-10 mg oral every 6-8 hours is frequently used as a muscle relaxant to treat acute back pain if it is believed to be from muscle spasm, though more recent data does refute this practice.20,21 Diazepam has a long half-life that is increased dramatically in the setting of repeat dosing. Diazepam also has sedative properties due to its inhibitory effect on the GABA-alpha receptors and can find additional uses in the field: management of agitation; temporizing withdrawal symptoms from alcohol, opioids, and benzodiazepines; temporizing the emergence reaction of ketamine. It can be administered PO/IV/IO/IM/PR at doses of 5-10 mg. However, due to the risks of respiratory depression in the field with inadequate airway supplies, extreme caution should be used when choosing benzodiazepines, and only once alternative options have been considered.
Ophthalmic Analgesics
Ocular pain from conjunctivitis, corneal abrasion, traumatic iritis, or photokeratitis (e.g., snow blindness) requires topical analgesia, commonly with tetracaine 0.5% ophthalmic drops given as 1-2 drops into the affected eye every 30 minutes. Prolonged use has not been shown to create a significant increase in adverse events, though some advocate for using a 10:1 dilution to minimize any potential harm.22,23 For cases of photokeratitis or traumatic iritis, cycloplegics, such as cyclopentolate 2% 1-2 drops every 8 hours, prevent ciliary body spasm and further pain. If cycloplegic drops are not available, a scopolamine (hyoscine) patch has been reported to create a similar analgesic effect.24,25 Cycloplegics will dilate the pupil and therefore lessen a patient’s visual acuity, which may be of concern if they are to be undertaking technically difficult tasks. Ophthalmic steroids such as dexamethasone 0.1% solution can be added for severe inflammation causing pain. Typical dosing is one drop every 1 hour titrated down to every 6 hours as tolerated.
Otic Analgesics
Otitis externa (OE, swimmer’s ear) benefits from direct administration of anti-inflammatory medications into the ear canal, commonly found in preparation with an antibiotic. A standard preparation is a ciprofloxacin/dexamethasone combination administered as 4 drops into the affected ear every 12 hours for seven days. An ear wick may also be coated with drops and used to facilitate distribution to all parts of the external canal surface area.26 Of note, the ophthalmic preparation of dexamethasone can also be used for otic conditions, given as 3-4 drops every 8-12 hours with any antibiotic, if indicated, administered separately. Injectable forms of anesthetic agents (i.e., bupivacaine, lidocaine) can be used for topical otic analgesia, delivered in 3 drop increments.27,28
Local/Regional Anesthesia
Direct injection of an anesthetic agent can provide fast and long-lasting relief without the potential side effects of systemic medications. Bupivacaine 0.25% with epinephrine (1:200,000) is ideal for local injection into laceration sites as well as hematoma blocks for fractures, dental blocks, regional blocks, and intra-articular injections for dislocation reduction or debilitating arthralgias. When performing a digital block, it is important to note that substantial evidence supports that epinephrine does not increase the risk of tissue/digital ischemia, but a ring-block or circular-block must be avoided, and care should be taken to limit total volume instilled (recommend <5 mL administration).29,30 Bupivacaine is preferred to lidocaine due to its longer duration (up to 8 hours versus 2 hours for lidocaine).31 A common misconception of bupivacaine that often limits its use is that is has a prolonged onset of action. In reality, its onset is roughly 2-10 minutes.29,32 Care should be taken to not administer an amount past the toxic dose, which is 3 mg/kg (with epinephrine).32 Epinephrine may further assist in hemostasis if the injury involves a laceration. Dexamethasone 4-6 mg IV can be co-administered to increase the duration of regional blocks.33-35 For patients with allergies to amide anesthetics and in the setting of a robust medical kit, the intravenous formulation of diphenhydramine (50 mg/mL) can be diluted to 1% (1 mL of diphenhydramine with 4 mL NS) and used in the same way as traditional local anesthetics.36 See the chapter on nerve blocks for further discussion on block safety and specific block anatomy.
Topical Anesthesia
Application of gauze soaked in bupivacaine or topical viscous lidocaine 2%, lidocaine 4-5% patch, lidocaine 3-5% cream or 5% ointment may provide direct analgesia to superficial wounds or burns. Viscous lidocaine can also be of benefit in patients with oral mucosal pain from ulcers or esophageal pain from gastroesophageal reflux disease (GERD) and in whom NSAIDs are contraindicated. Other topical formulations include EMLA (lidocaine + prilocaine), and LET (lidocaine-epinephrine-tetracaine). EMLA comes in a cream formulation and requires 45-60 min application prior to onset, whereas LET comes in a gel formulation and requires 30-45 min application prior to analgesic onset. Both may be applied to either intact skin or open wounds. Diclofenac, an NSAID, is available as a topical 1% gel applied every 6 hours or a 1.3% patch applied to the area every 12 hours.
Oral Opioids
For pain not controlled by the mentioned non-pharmacologic and non-opioid analgesic modalities and not better addressed by another form of pain control (i.e., local/regional anesthesia, ophthalmic drops, etc.), a clinician should consider oral (PO) opioids. Morphine sulfate immediate-release tablets at a dose of 15 mg every 4-6 hours or oral transmucosal fentanyl citrate (OTFC) 800 mcg every 15 minutes as needed may be preferable to the popular combination medications such as oxycodone, codeine, or hydrocodone plus acetaminophen.37,38 Buccal and sublingual fentanyl is also available, but OTFC is the preferred preparation and has the added potential benefit of being self-administered by the patient.8 Avoiding administration of the combination of opioid and acetaminophen – oxycodone/acetaminophen, hydrocodone/acetaminophen - allows for better opioid dose titration and helps limit the risk of administering toxic doses of acetaminophen. Opioids do have numerous side effects that are undesirable in the wilderness setting (confusion, delirium, vomiting, respiratory depression) and should, therefore, be given judiciously. PO morphine’s onset of action is 30 minutes with a duration of 3-5 hours, whereas the fentanyl preparations have an onset of 5-15 minutes and shorter durations. OTFC has a variable duration based on blood-levels since it has partial transmucosal absorption and partial, delayed GI tract absorption. This fact potentially favors its usage in the field.
Parenteral opioids
When the patient cannot tolerate oral medications for various reasons, consider intravenous (IV), intraosseous (IO), subcutaneous (SQ), and intranasal (IN) routes of administration. SQ and IN administration is preferable, as these do not require placement of an IV line or IO needle. Fentanyl is frequently used in this setting due to its rapid onset, short duration, and reduced effect on hemodynamics.39 Common dosing is 25-50 mcg IV/IO, 100-150 mcg IN, or 2-4 mcg/kg via nebulization every 30-60 minutes titrated to pain relief or appearance of adverse effect. However, due to fentanyl’s frequent need for redosing, morphine may be preferred due to its longer duration, particularly in the setting of a prolonged extraction or evacuation. Common dosing is 2.5–5 mg IV/IO/SQ (up to 0.1 mg/kg) every 4-6 hours in the opioid- naïve patient or nebulized at 20 mg per dose titrated to the analgesic effect. Note – IM administration has variable absorption, pain on injection, and prolonged onset of action and should not be used.
Note: If carrying and utilizing opioids or benzodiazepines, the medical kit should also contain appropriate reversal agents (naloxone, flumazenil). When administering medication via the intranasal route, it is best to use the most concentrated form available; otherwise, the large amount of liquid will saturate the mucosal surface area, and much will not be absorbed. Similarly, smaller aliquots of a large dose can be given in rapidly titratable fashion.
Steroids
Steroids can be beneficial for managing the pain of various etiologies: acute, chronic, neuropathic, inflammatory.40-43 When more traditional analgesic modalities are failing to adequately control pain or when a more rapid functional recovery is required, consider adding dexamethasone 4 mg PO every 6 hours as an analgesic adjunct.
Natural/Herbal/Home Remedy Analgesics
There are several other non-pharmacologic pain remedies described anecdotally in the literature, which may be useful if available. Ginger and turmeric have both been touted for their analgesic & anti-inflammatory properties.44-46 Honey, boiled potato skins, and boiled banana leaves are helpful in burn treatment when applied directly to the site, with honey offering the additional benefit of having anti-pseudomonal properties.47 Topical aloe vera has long been a remedy for sunburn and superficial thermal injuries.48,49 For ear pain, a 1:1 solution of white wine vinegar with water or a 1:1 lemon juice to water solution may be applied to ears afflicted with OE or before prolonged water submersion to prevent OE.50 Myrtle rouge (red myrtle) is an essential oil used by some divers applied to the posterior auricular region and the entrance to the auditory canal to decrease otic pain and inflammation from frequent and prolonged submersion.
CAVEATS
When considering the use of regional blocks or cycloplegics (as well as any medication with effects on the central nervous system), be aware of how it will limit the patient’s ability to participate in their own evacuation/extraction. Benzodiazepines and opioids may sedate a patient and impair ambulation. An ophthalmic cycloplegic may disrupt their vision to the point that they cannot navigate simple terrain on their own. Likewise, an extremity block may impair a patient’s limb use due to loss of motor-sensory function. Every decision in the wild can have significant consequences, not commonly thought of in the ED.
DISPOSITION
Many of the painful conditions treated in the wild can be dealt with in the field and will not require evacuation. However, if the etiology and diagnosis are such that evacuation is in the best interest of the patient, the physician should recommend such.
MEDICAL KIT
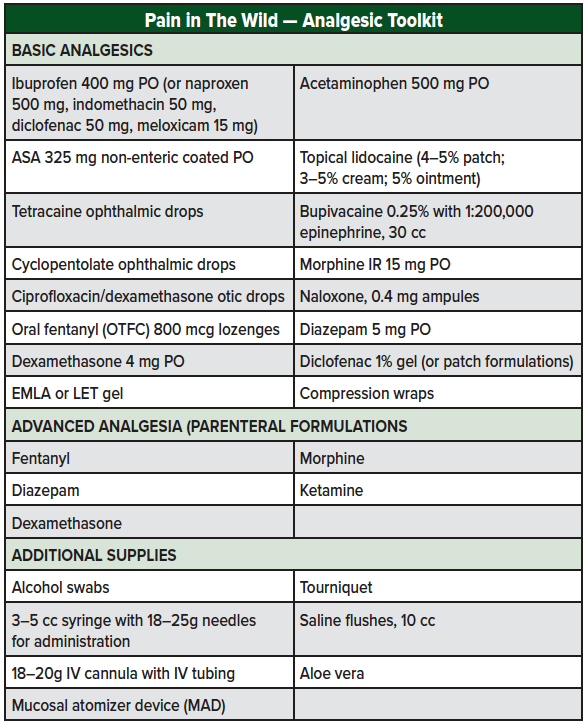
There is no "perfect" medical kit for working or traveling outdoors, but the following items provide a foundation for addressing pain in the wilderness setting. More basic kits will focus on oral and topical applications, whereas an advanced kit will have the ability to escalate care to injectable routes. Choosing medications with multiple potential functions (eg, dexamethasone for altitude illness as well as pain control) and preparations of those medications that work to decrease size and weight is ideal (eg, choosing 400 mg ibuprofen tablets over 200 mg). Utilization of injectable, IV, or IN medications will require additional materials such as syringes, needles, atomizers, or IV "start-kits" and tubing. The author recommends utilizing oral, topical, or local/regional administration routes first and then escalating to IV, SQ, or IN as needed. The IV/IO approach should be reserved for extreme injuries/situations where evacuation to advanced care is required/planned or when additional medications are deemed necessary (eg, IV fluids) and only among trained and experienced users.
References
- Ziesler, P. Statistical abstract: 2018. Natural Resource Data Series. NPS/NRSS/EQD/NRDS—2019/1219. National Park Service. Fort Collins, Colorado. 2018; Available from: https://irma.nps.gov/DataStore/Reference/Profile/2259799.
- Heggie, T.W. and M.E. Amundson, Dead men walking: search and rescue in US National Parks. Wilderness Environ Med, 2009. 20(3): p. 244-9.
- Search and rescue incidents for 2017. Operations Dashboard: US National Park Service. Accessed September 25, 2019. 2017.
- Flores, A.H., T. Haileyesus, and A.I. Greenspan, National estimates of outdoor recreational injuries treated in emergency departments, United States, 2004-2005. Wilderness Environ Med, 2008. 19(2): p. 91-8.
- Griffin, B.R., et al., Cool Running Water First Aid Decreases Skin Grafting Requirements in Pediatric Burns: A Cohort Study of Two Thousand Four Hundred Ninety-five Children. Ann Emerg Med, 2019.
- Busch, V., et al., The effect of deep and slow breathing on pain perception, autonomic activity, and mood processing--an experimental study. Pain Med, 2012. 13(2): p. 215-28.
- Chang, A.K., et al., Effect of a Single Dose of Oral Opioid and Nonopioid Analgesics on Acute Extremity Pain in the Emergency Department: A Randomized Clinical Trial. JAMA, 2017. 318(17): p. 1661-1667.
- Butler, F.K., et al., A Triple-Option Analgesia Plan for Tactical Combat Casualty Care: TCCC Guidelines Change 13-04. J Spec Oper Med, 2014. 14(1): p. 13-25.
- Derry, S. and R.A. Moore, Single dose oral aspirin for acute postoperative pain in adults. Cochrane Database Syst Rev, 2012(4): p. CD002067.
- Diener, H.C., M. Gold, and M. Hagen, Use of a fixed combination of acetylsalicylic acid, acetaminophen and caffeine compared with acetaminophen alone in episodic tension-type headache: meta-analysis of four randomized, double-blind, placebo-controlled, crossover studies. J Headache Pain, 2014. 15: p. 76.
- Goldstein, J., et al., Acetaminophen, aspirin, and caffeine in combination versus ibuprofen for acute migraine: results from a multicenter, double-blind, randomized, parallel-group, single-dose, placebo-controlled study. Headache, 2006. 46(3): p. 444-53.
- Le Parc, J.M., et al., Comparative tolerability of paracetamol, aspirin and ibuprofen for short-term analgesia in patients with musculoskeletal conditions: results in 4291 patients. Clin Rheumatol, 2002. 21(1): p. 28-31.
- Motov, S., et al., A prospective randomized, double-dummy trial comparing IV push low dose ketamine to short infusion of low dose ketamine for treatment of pain in the ED. Am J Emerg Med, 2017. 35(8): p. 1095-1100.
- Jennings, P.A., et al., Morphine and ketamine is superior to morphine alone for out-of-hospital trauma analgesia: a randomized controlled trial. Ann Emerg Med, 2012. 59(6): p. 497-503.
- Lawthaweesawat, C., et al., Prehospital Care of the 13 Hypothermic, Anesthetized Patients in the Thailand Cave Rescue. N Engl J Med, 2019. 380(14): p. 1372-1373.
- Poonai, N., et al., Intranasal ketamine for procedural sedation and analgesia in children: A systematic review. PLoS One, 2017. 12(3): p. e0173253.
- Guthrie, A.M., et al., Use of Intranasal Ketamine in Pediatric Patients in the Emergency Department. Pediatr Emerg Care, 2019.
- Rosenbaum, S.B. and J.L. Palacios, Ketamine, in StatPearls. 2019: Treasure Island (FL).
- Green, S.M., et al., Clinical practice guideline for emergency department ketamine dissociative sedation: 2011 update. Ann Emerg Med, 2011. 57(5): p. 449-61.
- van Tulder, M.W., et al., Muscle relaxants for nonspecific low back pain: a systematic review within the framework of the cochrane collaboration. Spine (Phila Pa 1976), 2003. 28(17): p. 1978-92.
- Friedman, B.W., et al., Diazepam Is No Better Than Placebo When Added to Naproxen for Acute Low Back Pain. Ann Emerg Med, 2017. 70(2): p. 169-176 e1.
- Waldman, N., I.K. Densie, and P. Herbison, Topical tetracaine used for 24 hours is safe and rated highly effective by patients for the treatment of pain caused by corneal abrasions: a double-blind, randomized clinical trial. Acad Emerg Med, 2014. 21(4): p. 374-82.
- Ball, I.M., et al., Dilute proparacaine for the management of acute corneal injuries in the emergency department. CJEM, 2010. 12(5): p. 389-96.
- Hannon, B., et al., Transdermal hyoscine induced unilateral mydriasis. BMJ Case Rep, 2012. 2012.
- Shah, J., A. Jiang, and Z. Fekete, Anisocoria secondary to inadvertent contact with scopolamine patch. BMJ Case Rep, 2017. 2017.
- Potter, M. Effective Treatment for Acute Otitis Externa. 2016; Available from: https://contemporaryclinic.pharmacytimes.com/journals/issue/2016/june2016/effective-treatment-for-acute-otitis-externa/p-2.
- Prasad, S. and B. Ewigman, Use anesthetic drops to relieve acute otitis media pain. J Fam Pract, 2008. 57(6): p. 370-3.
- Bolt, P., et al., Topical lignocaine for pain relief in acute otitis media: results of a double-blind placebo-controlled randomised trial. Arch Dis Child, 2008. 93(1): p. 40-4.
- Wilhelmi, B.J., et al., Do not use epinephrine in digital blocks: myth or truth? Plast Reconstr Surg, 2001. 107(2): p. 393-7.
- Chowdhry, S., et al., Do not use epinephrine in digital blocks: myth or truth? Part II. A retrospective review of 1111 cases. Plast Reconstr Surg, 2010. 126(6): p. 2031-4.
- Barash P.G., B.F. Cullen, R.K. Stoelting, et al., eds. Clinical Anesthesia 8th ed. Philadelphia, PA: Wolters Kluwer; 2017: p. 564-580.
- Bupivacaine (package insert). 2011 Dec 11, 2019; Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/018692s015lbl.pdf.
- Vieira, P.A., et al., Dexamethasone with bupivacaine increases duration of analgesia in ultrasound-guided interscalene brachial plexus blockade. Eur J Anaesthesiol, 2010. 27(3): p. 285-8.
- Heesen, M., et al., Co-administration of dexamethasone with peripheral nerve block: intravenous vs perineural application: systematic review, meta-analysis, meta-regression and trial-sequential analysis. Br J Anaesth, 2018. 120(2): p. 212-227.
- Tandoc, M.N., et al., Adjuvant dexamethasone with bupivacaine prolongs the duration of interscalene block: a prospective randomized trial. J Anesth, 2011. 25(5): p. 704-9.
- Ernst, A.A., et al., 1% lidocaine versus 0.5% diphenhydramine for local anesthesia in minor laceration repair. Ann Emerg Med, 1994. 23(6): p. 1328-32.
- Van Dyke, T., et al., Combination oxycodone 5 mg/ibuprofen 400 mg for the treatment of postoperative pain: a double-blind, placebo- and active-controlled parallel-group study. Clin Ther, 2004. 26(12): p. 2003-14.
- Herring, A.A., M.L. Kent, and M. Young, Auerbach’s Wilderness Medicine, 7th edition. Chapter 47 Principles of Pain Management. . 2016.
- Kanowitz, A., et al., Safety and effectiveness of fentanyl administration for prehospital pain management. Prehosp Emerg Care, 2006. 10(1): p. 1-7.
- Watanabe, S. and E. Bruera, Corticosteroids as adjuvant analgesics. J Pain Symptom Manage, 1994. 9(7): p. 442-5.
- Li, D., et al., Effect of Intravenous Corticosteroids on Pain Management and Early Rehabilitation in Patients Undergoing Total Knee or Hip Arthroplasty: A Meta-Analysis of Randomized Controlled Trials. Pain Pract, 2018. 18(4): p. 487-499.
- Bergkvist, P.I. and K. Sjobeck, Antibiotic and prednisolone therapy of erysipelas: a randomized, double blind, placebo-controlled study. Scand J Infect Dis, 1997. 29(4): p. 377-82.
- Stevens, D.L., et al., Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of America. Clin Infect Dis, 2014. 59(2): p. 147-59.
- Altman, R.D. and K.C. Marcussen, Effects of a ginger extract on knee pain in patients with osteoarthritis. Arthritis Rheum, 2001. 44(11): p. 2531-8.
- Bartels, E.M., et al., Efficacy and safety of ginger in osteoarthritis patients: a meta-analysis of randomized placebo-controlled trials. Osteoarthritis Cartilage, 2015. 23(1): p. 13-21.
- Daily, J.W., M. Yang, and S. Park, Efficacy of Turmeric Extracts and Curcumin for Alleviating the Symptoms of Joint Arthritis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J Med Food, 2016. 19(8): p. 717-29.
- Bitter, C.C. and T.B. Erickson, Management of Burn Injuries in the Wilderness: Lessons from Low-Resource Settings. Wilderness Environ Med, 2016. 27(4): p. 519-525.
- Maenthaisong, R., et al., The efficacy of aloe vera used for burn wound healing: a systematic review. Burns, 2007. 33(6): p. 713-8.
- Luo, X., et al., Aloin Suppresses Lipopolysaccharide-Induced Inflammatory Response and Apoptosis by Inhibiting the Activation of NF-kappaB. Molecules, 2018. 23(3).
- Thalmann ED. DAN revisits and expands on the preventive measures for otitis externa. 1999 Dec 11, 2019]; Available from: https://www.diversalertnetwork.org/medical/articles/More_On_Swimmers_Ear.





